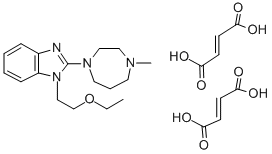
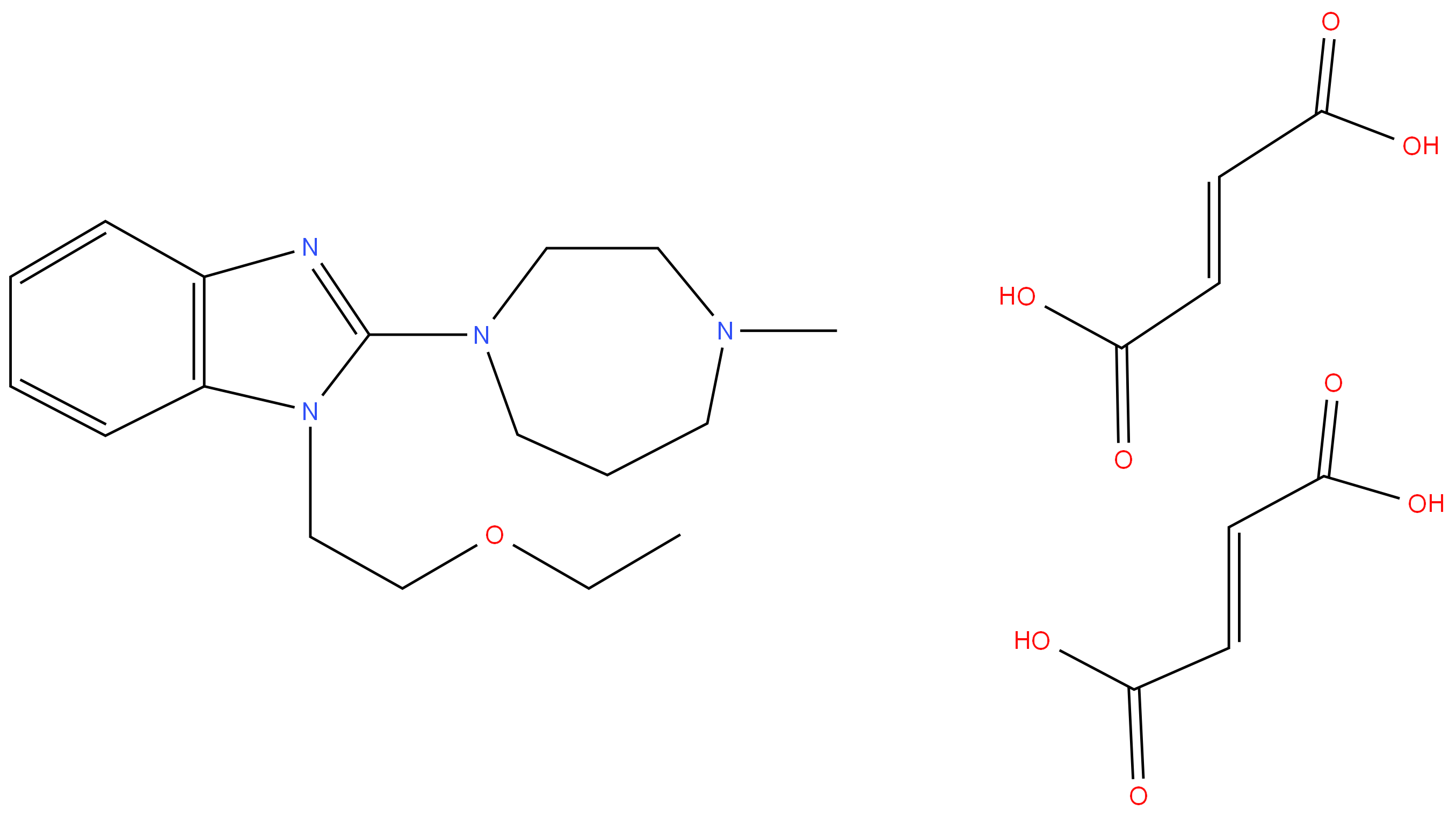
EMEDASTINE FUMARATE
- CAS NO.:87233-62-3
- Empirical Formula: C25H34N4O9
- Molecular Weight: 534.56
- MDL number: MFCD00923844
- EINECS: 805-825-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-15 15:30:59
What is EMEDASTINE FUMARATE?
Description
Emedastine is a histamine H1 receptor antagonist (Ki = 1.3 nM). It is selective for histamine H1 over H2 and H3 receptors (Kis = 49 and 12.43 μM, respectively), as well as α1-, α2-, and β1-adrenergic and dopamine D1 and D2 receptors, and the serotonin (5-HT) receptor subtypes 5-HT1 and 5-HT2 at 10 μM. Emedastine inhibits histamine-induced phosphoinositide turnover and intracellular calcium mobilization in primary human conjunctival epithelial cells (HCECs; IC50s = 1.6 and 2.9 nM, respectively). It also inhibits histamine-stimulated secretion of IL-6, IL-8, and GM-CSF by primary HCECs (IC50s = 2.23, 3.42, and 1.50 nM, respectively). Ocular application of emedastine prior to histamine challenge inhibits vascular permeability in guinea pigs. Formulations containing emedastine have been used in the treatment of allergic conjunctivitis.
Description
Emedastine difumarate, a potent H1-receptor antagonist, was launched in Japan for the treatment of allergic rhinitis and urticaria. Emedastine exerts its antiallergic effect via inhibition of substance P-induced histamine release. It has been demonstrated both in vitro and in vivo that this effect is mediated by the inhibition of Ca2+release from extracellular stores and of Ca2+ influx into mast cells. In a clinical trial with bronchial asthma, emedastine improved asthmatic symptoms in 55.3% of patients.
Chemical properties
White or yellowish powder
Originator
Kanebo (Japan)
The Uses of EMEDASTINE FUMARATE
Antihistaminic, H1-receptor; asthma prophylactic; anti-allergic.
Definition
ChEBI: The fumaric acid salt of emedastine containing two molecules of fumaric acid for each molecule of emedastine. A relatively selective histamine H1 antagonist, it is used for allergic rhinitis, urticaria, and pruritic skin disorders, and in eyedrops for the symptomatic relief of allergic conjuntivitis.
Manufacturing Process
Preparation of 2-(4-methyl-1-piperazinyl)benzimidazole. A mixture of 2-
chlorobenzimidazole (10.00 g) and N-mehylpiperazine (20.00 g) is stirred at
125°C for 5 hours. A 10% aqueous sodium hydroxide (100 ml) is added to the
reaction mixture, and the precipitated crystals are separated by filtration. The
filtrate is extracted with chloroform, and the chloroform extract is evaporated
to dryness to give the same crystals. The crystals are combined and
recrystallized from water-methanol to give 2-(4-methyl-1-
piperazinyl)benzimidazole (7.02 g) as colorless needles, m.p. 225°-226°C.
2-(4-Methyl-1-piperazinyl)benzimididazole (5.00 g) prepared as above is
dissolved in N,N-dimethylformamide (50 ml) and thereto is added sodium
hydride (concentration: 50%) (1.50 g) at room temperature, and the mixture
is stirred for 30 minutes. To the mixture is added 2-bromoethyl ethyl ether
(4.00 g), and the mixture is stirred at 70°C for 10 hours. To the reaction
mixture is added water (150 ml), and the mixture is extracted with ethyl
acetate. The extract is washed with water, dried over anhydrous magnesium
sulfate and then concentrated to give a brown oily substance (5.40 g). The
brown oily substance is treated with fumaric acid (3.26 g) in hot ethanol. The
crude crystals thus obtained are recrystallized from ethyl acetate-ethanol to
give 1-[2-(ethoxy)ethyl]-2-(4-methyl-1-piperazinyl)benzimidazole 3/2
fumarate (6.31 g) as colorless plates, melting point 167.5°-168.5°C.
Elementary analysis for C22H30N4O7: Calcd. (%): C, 57.13; H, 6.54; N, 12.11;
Found (%): C, 57.04; H, 6.44; N, 12.02.
1-[2-(Ethoxy)ethyl]-2-(4-methyl-1-piperazinyl)benzimidazole can be prepared
using 2-chloro-(1-[2-(ethoxy)ethyl]benzimidazole), (last one can be produced
from 2-bromoethyl ethyl ether 2-chlorobenzimidazole) and N-methylpiperazine
and fumaric acid there are obtained crude crystals, which are recrystallized
from ethanol to give 1-[2-(ethoxy)ethyl]-2-(4-methyl-1-
piperazinyl)benzimidazole 3/2 fumarate. This product has the same physical
properties as those of the product above described.
brand name
Emadine (Alcon);Daren;Remicut.
Therapeutic Function
Antiallergic, Antihistaminic
Hazard
A poison by ingestion.
Properties of EMEDASTINE FUMARATE
| Melting point: | 148-151° |
| storage temp. | 2-8°C |
| solubility | Soluble in water, sparingly soluble in anhydrous ethanol, very slightly soluble in acetone. |
| form | neat |
| Merck | 14,3557 |
Safety information for EMEDASTINE FUMARATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P501:Dispose of contents/container to..… |
Computed Descriptors for EMEDASTINE FUMARATE
EMEDASTINE FUMARATE manufacturer
BDR Pharmaceuticals International Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran
You may like
-
 87233-62-3 Emedastine difumarate 98%View Details
87233-62-3 Emedastine difumarate 98%View Details
87233-62-3 -
 Emedastine difumarate 98% CAS 87233-62-3View Details
Emedastine difumarate 98% CAS 87233-62-3View Details
87233-62-3 -
 Emedastine difumarate CAS 87233-62-3View Details
Emedastine difumarate CAS 87233-62-3View Details
87233-62-3 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5