Elbasvir
- CAS NO.:1370468-36-2
- Empirical Formula: C49H55N9O7
- Molecular Weight: 882.02
- MDL number: MFCD28124343
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
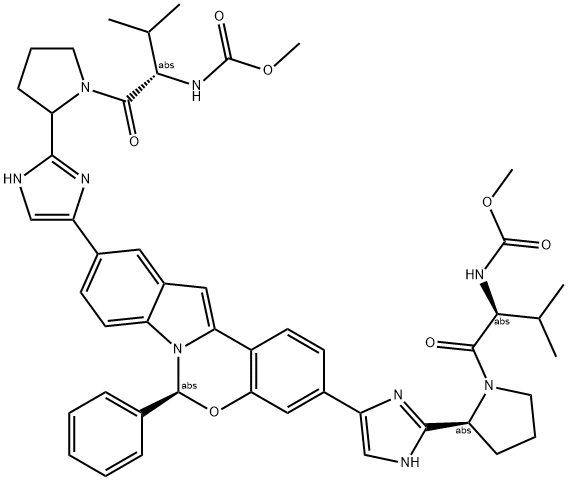
What is Elbasvir?
Absorption
Elbasvir reaches peak plasma concentration 3-6 hours after administration and has an absolute bioavailability of 32%. When co-administered with food, the peak concentration of elbasvir increases 1.5-fold, but this increase in exposure is not likely to be clinically relevant.
Toxicity
The most commonly reported adverse reactions of all intensity (greater than or equal to 5% in placebo-controlled trials) were fatigue, headache, and nausea.
The Uses of Elbasvir
Elbasvir is a pharmaceutical drug that is used in the treatment of hepatitis C virus in patients with HCV genotype 1 infection. It inhibits an important viral phosphoprotein, NS5A, which is involved in viral replication, assembly, and secretion.
Background
Elbasvir is a direct-acting antiviral medication used as part of combination therapy to treat chronic hepatitis C, an infectious liver disease caused by infection with hepatitis C virus (HCV). HCV is a single-stranded RNA virus that is categorized into nine distinct genotypes, with genotype 1 being the most common in the United States, affecting 72% of all chronic HCV patients. Treatment options for chronic hepatitis C have advanced significantly since 2011, with the development of direct-acting antivirals (DAAs) such as elbasvir. Elbasvir is an inhibitor of NS5A, a protein essential for viral replication and virion assembly. The barrier to the development of resistance to NS5A inhibitors is lower than that of NS5B inhibitors, another class of DAAs. Substitutions at amino acid positions 28, 30, 31, or 93 are known to confer resistance to elbasvir. Despite this disadvantage elbasvir is still effective against HCV, particularly when paired with grazoprevir.
Elbasvir is available as a fixed-dose combination product with grazoprevir (tradename: Zepatier) used for the treatment of chronic hepatitis C. Approved in January 2016 by the FDA, Zepatier is indicated for the treatment of HCV genotypes 1 and 4 with or without ribavirin depending on the presence of resistance-associated amino acid substitutions in the NS5A protein and previous treatment failure with ribavirin, peginterferon alfa-2a, peginterferon alfa-2b, or other NS3/4A inhibitors like boceprevir, simeprevir, or telaprevir. Elbasvir and grazoprevir are used with or without ribavirin with the intent to cure, or achieve a sustained virologic response (SVR), and have been shown to achieve a SVR between 94% and 97% for genotype 1 and 97% and 100% for genotype 4 after 12 weeks of treatment.. SVR and eradication of HCV infection are associated with significant long-term health benefits including reduced liver-related damage, improved quality of life, reduced incidence of hepatocellular carcinoma, and reduced all-cause mortality.
In a computational target-based drug repurposing investigation published in April 2020, elbasvir was predicted to bind stably and preferentially to three proteins necessary for viral replication of SARS-CoV-2, the human coronavirus responsible for the COVID-19 pandemic. While these results are suggestive of antiviral efficacy, follow-up clinical trials are required to validate elbasvir as a potential therapy against SARS-CoV-2.
Indications
Elbasvir, when used in combination with grazoprevir as the combination product Zepatier, is indicated for use with or without ribavirin for the treatment of chronic HCV genotypes 1 or 4 infection in adults.
Definition
ChEBI: A complex organic heterotetracyclic compound that is a hepatitis C virus nonstructural protein 5A inhibitor used in combination with grazoprevir (under the brand name Zepatier) for treatment of chronic HCV genotypes 1 or 4 infection in adults.
Pharmacokinetics
Elbasvir is classified as a direct-acting antiviral (DAA) and prevents viral replication in HCV genotypes 1a, 1b, and 4.
Metabolism
Elbasvir is partially eliminated by oxidative metabolism meditated by CYP3A. No circulating metabolites of elbasvir have been detected in human plasma.
Properties of Elbasvir
| Density | 1.40±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMF: 33 mg/ml; DMSO: 20 mgm/l; Ethanol: 33 mg/ml; Ethanol:PBS (pH 7.2)(1:2): 0.33 mg/ml |
| form | A crystalline solid |
| pka | 11.22±0.46(Predicted) |
| color | White to yellow |
Safety information for Elbasvir
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H226:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P243:Take precautionary measures against static discharge. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P370+P378:In case of fire: Use … for extinction. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for Elbasvir
Related products of tetrahydrofuran
You may like
-
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 -
 99-30-9 99%View Details
99-30-9 99%View Details
99-30-9 -
 104944-18-5 99%View Details
104944-18-5 99%View Details
104944-18-5 -
 3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
37951-49-8 -
 98-56-6 99%View Details
98-56-6 99%View Details
98-56-6 -
 694-48-4 99%View Details
694-48-4 99%View Details
694-48-4 -
 51364-51-3 99%View Details
51364-51-3 99%View Details
51364-51-3 -
 Methyl chloroacetate, 98% 96-34-4 99%View Details
Methyl chloroacetate, 98% 96-34-4 99%View Details
96-34-4