Elamipretide
- CAS NO.:736992-21-5
- Empirical Formula: C32H49N9O5
- Molecular Weight: 639.79
- MDL number: MFCD20133791
- EINECS: 205-858-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-23 19:56:12
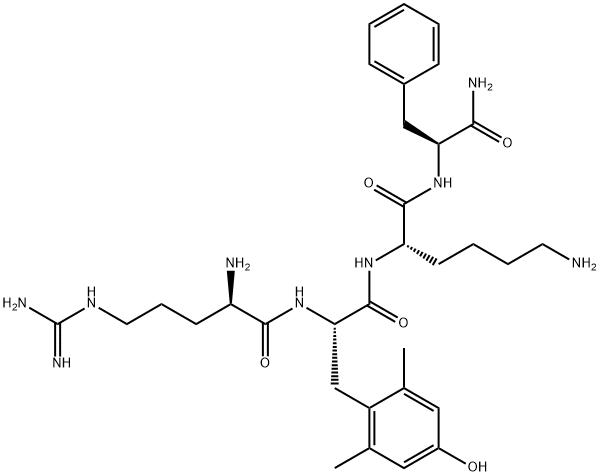
What is Elamipretide?
Description
Elamipretide, also known as MTP 131, is a mitochondria-targeted peptide antioxidant. Elamipretide decreases inflammation and protect against a variety of neurological illnesses. Elamipretide (SS-31) improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in mice. Elamipretide Promotes Mitophagosome Formation and Prevents Its Reduction Induced by Nutrient Excess in INS1 β-cells.
The Uses of Elamipretide
D-Arginyl-2,6-dimethyl-L-tyrosyl-L-lysyl-L-phenylalaninamide-d5 Trifluoroacetic Acid Salt is labelled D-Arginyl-2,6-dimethyl-L-tyrosyl-L-lysyl-L-phenylalaninamide (A769700) which is a mitochondrial-targeted compound that re-energizes ischemic mitochondria by interacting with cardiolipin. It is a cell-permeable antioxidant peptide.
Mechanism of action
Elamipretide can inhibit the production of pathogenic ROS (reactive oxygen species) and restore mitochondrial ATP synthesis. Currently, the most accepted mechanism for these two actions is the direct interaction between Elamipretide and cardiolipin.
Clinical Use
Barth syndrome is an ultra-rare genetic condition characterized by cardiac abnormalities leading to exercise intolerance, muscle weakness, debilitating fatigue, heart failure, recurrent infections, and delayed growth. There are currently no FDA- or EMA-approved therapies for patients with Barth syndrome. Elamipretide has an Orphan Drug, Fast Track and Rare Pediatric Designation from the FDA and Orphan Drug Designation from the EMA for treating Barth syndrome.
Side Effects
Side effects of elamipretide include:
Mild redness or itching at the injection site
Headache
Dizziness
Properties of Elamipretide
| Density | 1.34±0.1 g/cm3(Predicted) |
| solubility | Methanol (Slightly), Water (Slightly) |
| pka | 10.10±0.25(Predicted) |
| form | Solid |
| color | White to Off-White |
| Stability: | Hygroscopic |
Safety information for Elamipretide
Computed Descriptors for Elamipretide
| InChIKey | SFVLTCAESLKEHH-WKAQUBQDSA-N |
| SMILES | C(N)(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC1=C(C)C=C(O)C=C1C)NC(=O)[C@@H](CCCNC(N)=N)N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8