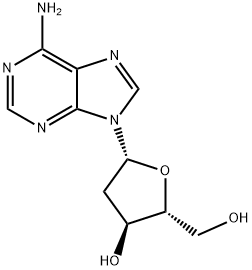
2'-Deoxyadenosine
- CAS NO.:958-09-8
- Empirical Formula: C10H13N5O3
- Molecular Weight: 251.24
- MDL number: MFCD00005754
- EINECS: 213-488-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
What is 2'-Deoxyadenosine ?
Description
2'-Deoxyadenosine is a natural deoxyribonucleoside, which is a structural fragment of deoxyribonucleic acid DNA. It is a purine nucleoside component of DNA comprised of adenosine linked by its N9 nitrogen to the C1 carbon of deoxyribose. Like other natural nucleosides, it is involved in the transmission of genetic information in almost all biological cells, affecting protein synthesis and polysaccharide. It plays a very important role in regulating the growth, proliferation, differentiation and inhibition of cells in vivo.
The Uses of 2'-Deoxyadenosine
2'-Deoxyadenosine is used in paradoxical stimulation study of human sperm motility. It plays an essential role as sperm motility stimulants used in sperm micro-injection on mouse and human embryo development. It finds an application in some cells as an energy source under energy stress conditions. It is involved in various biological processes to compare the functions of adenosine analogues.
What are the applications of Application
2′-Deoxyadenosine is a nucleoside adenosine derivative with mutagenic properties
Definition
ChEBI: 2'-deoxyadenosine is a purine 2'-deoxyribonucleoside having adenine as the nucleobase. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a purines 2'-deoxy-D-ribonucleoside and a purine 2'-deoxyribonucleoside.
Preparation
2'-deoxyadenosine synthesis: esterification of adenosine, acylation of an acylating agent and an acid binding agent to obtain an acylate; the obtained acylate is then subjected to reduction and purification to obtain 2'-deoxyadenosine. The esterification agent used in the esterification process is dialkyl tin oxide, 2'-O-p-toluenesulfonyladenosine is synthesized from adenosine, and 2'-deoxyadenosine is synthesized from 2'-O-p-toluenesulfonyladenosine glycosides.
Biological Functions
2'-Deoxyadenosine (Deoxyadenosine) is a critical component of DNA. When present in sufficiently high levels, deoxyadensoine can act as an immunotoxin and a metabotoxin. An immunotoxin disrupts, limits the function, or destroys immune cells. A metabotoxin is an endogenous metabolite that causes adverse health effects at chronically high levels.
Biological Activity
2'-Deoxyadenosine is a deoxyribonucleoside and an intermediate in the purine nucleotide degradation pathway. It is transported into cells via facilitated diffusion or formed within cells by degradation of S-adenosylhomocysteine or AMP and is removed from cells by purine metabolism or is converted into adenine nucleotides. 2'-Deoxyadenosine has been used in the characterization of DNA conformations and the synthesis of nucleoside analogs as antiviral agents.
Source
2'-Deoxyadenosine is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). 2'-Deoxyadenosine is a natural product found in Streptomyces jiujiangensis, Fritillaria przewalskii, and other organisms with data available.
Properties of 2'-Deoxyadenosine
| Melting point: | 187-189 °C |
| Boiling point: | 394.4°C (rough estimate) |
| Density | 1.2938 (rough estimate) |
| refractive index | 1.7610 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Slightly) |
| form | powder |
| pka | pK1:3.8(+1) (25°C,μ=0.1) |
| color | White |
| Water Solubility | Soluble in water and caustic soda. |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C10H13N5O3/c11-9-8-10(13-3-12-9)15(4-14-8)7-1-5(17)6(2-16)18-7/h3-7,16-17H,1-2H2,(H2,11,12,13)/t5-,6+,7+/m0/s1 |
| CAS DataBase Reference | 958-09-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Deoxyadenosine(958-09-8) |
| EPA Substance Registry System | Adenosine, 2'-deoxy- (958-09-8) |
Safety information for 2'-Deoxyadenosine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for 2'-Deoxyadenosine
| InChIKey | OLXZPDWKRNYJJZ-RRKCRQDMSA-N |
| SMILES | OC[C@H]1O[C@@H](N2C3C(=C(N=CN=3)N)N=C2)C[C@@H]1O |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
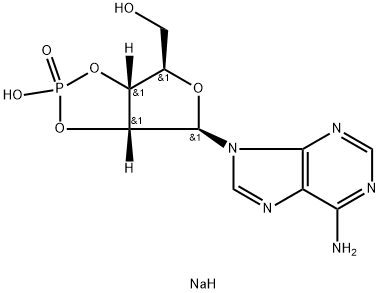
![[5-(6-aminopurin-9-yl)-4-hydroxy-3-phosphonooxy-oxolan-2-yl]methoxyphosphonic acid](https://img.chemicalbook.in/CAS/GIF/1053-73-2.gif)

You may like
-
 2'-Deoxyadenosine Anhydrous CAS 958-09-8View Details
2'-Deoxyadenosine Anhydrous CAS 958-09-8View Details
958-09-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8