Doramectin
Synonym(s):25-Cyclohexyl-5-O-demethyl-25-de(1-methylpropyl)avermectin;Doramectin
- CAS NO.:117704-25-3
- Empirical Formula: C50H74O14
- Molecular Weight: 899.11
- MDL number: MFCD00894747
- EINECS: 601-490-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-27 17:31:30
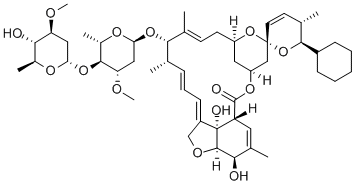
What is Doramectin?
Description
Doramectin is a novel avermectin with a cyclohexyl
substituent on the previously inaccessible C-25 position
of the molecule; the substitution was facilitated by
mutational biosynthesis. The discovery of doramectin
involved feeding carboxylic acids or their precursors to
a fermentation of Streptomyces avermitilis that lacked
branched-chain 2-oxo acid dehydrogenase activity, and
then screening the resultant fermentation products for
anthelmintic and ectoparasiticidal activity (89).
Two dosage forms of doramectin have been developed
for use as an endectocide on cattle, an injectable
formulation for subcutaneous administration and a pouron
formulation. An injectable formulation is also approved
for use on pigs.
Chemical properties
White solid or slightly yellow-brown powder with poor water solubility, low stability, easily decomposed and deactivated by sunlight. The remaining drug residue is toxic to fish and aquatic organisms, requiring water source protection.
The Uses of Doramectin
Doramectin is a biosynthetic avermectin derived from a mutant strain of Streptomyces avermitilis, supplemented with a cyclohexylcaboxylic acid starting unit. Doramectin was developed as an anthelmintic for internal parasite control. The presence of the cyclohexyl group replacing the sec-butyl moiety affords greater hydrophobicity and longer biological half-life compared to avermectin. Like the other milbemycin/avermectins, it selectively binds to parasite glutamate-gated chloride ion channels and disrupts neurotransmission leading to paralysis and death of the parasite.
What are the applications of Application
Doramectin is a mutational biosynthetic antiparasitic antibiotic structurally related to the avermectins. Avermectins are a group of agents that occurs naturally as a product of fermenting Streptomyces avermitilis, isolated from the soil. Avermectins are composed of a structurally similar 16-membered macrocyclic lactone, and widely used as insecticidal and antiparasitic agents. Doramectin is one of the best of a series of avermectins with antiparasitic activity.
What are the applications of Application
Doramectin is a mutational biosynthetic antiparasitic antibiotic
Pharmacokinetics
Doramectin is used for the treatment of livestock diseases such as nematodes and mites ectoparasitosis,it is fermented by recombinant avermitilis (Streptomyces avermitilis) new strain, and the main difference from ivermectin is that C25 is substituted by cyclohexyl. It is a new, broad-spectrum antiparasitic drug,and is efficient for gastrointestinal nematodes, lung nematodes, Euglena, lice, ticks, mites and wound maggots , also has a good effect on internal and external parasites especially certain nematodes (round worms) and arthropods , but invalid to tapeworms, flukes and protozoa.
Veterinary Drugs and Treatments
Doramectin injection is indicated for the treatment and control of
the following endo- and ectoparasites in cattle: roundworms (adults
and some fourth stage larvae)—Ostertagia ostertagi
(including inhibited
larvae), O. lyrata, Haemonchus placei, Trichostrongylus axei,
T. colubriformis,
T. longispicularis, Cooperia oncophora, C. pectinata,
C. punctata, C. surnabada (syn. mcmasteri),
Bunostomum phlebotomum,
Strongyloides papillosus, Oesophagostomum radiatum,
Trichuris spp.; lungworms (adults and fourth stage larvae)—Dictyocaulus
viviparus; eyeworms (adults)—Thelazia
spp.; grubs (parasitic
stages)—Hypoderma bovis, H. lineatum; lice—Haematopinus
eurysternus,
Linognathus vituli, Solenopotes capillatus; and mange
mites—Psoroptes bovis, Sarcoptes scabiei.
In swine the injection is labeled for the treatment and control
gastrointestinal roundworms (adults and 4th stage Ascaris suum,
adults and 4th stage Oesophagostomum dentatum, Oesophagostomum
quadrispinolatum adults, Strongyloides ransomi adults, and
Hydrostrongylus rubidus adults), lungworms (Stephanurus dentatus
adults), mange mites (adults and immature stages Sarcoptes
scabeii var. suis), and sucking lice (adults and immature stages
Haematopinus suis)
The manufacturer states the doramectin protects cattle against
infection or reinfection with Ostertagia ostertagi for up to 21 days.
Doramectin topical (pour-on) is approved for use in cattle and
has a similar spectrum of action against a variety of endo- and ectoparasites,
including biting lice.
Injectable doramectin has been used for treating a variety of
nematode and arthropod parasites in companion animals, including
generalized demodicosis in dogs and cats and spirocercosis in
dogs.
Structure and conformation
Doramectin is a macrolide drug of the avermectin family, which is very similar in structure to ivermectin. Doramectin C25 is cyclohexane, and ivermectin is a mixture of several homologues. The C25 of its highly active component B1a (80%) is CH(CH3)C2H5, and the C25 of another component B1b (20%) is CH(CH3)-CH3. Doramectin has a double bond between C22 and C23, while ivermectin has a single bond.
Mode of action
Its mechanism of action is to increase the release of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) of the worm, thereby blocking the transmission of nerve signals, so that muscle cells lose their ability to contract, resulting in death of the parasites. the neurotransmitter of Peripheral nerves of mammals is acetylcholine,and will not be affected by Doramectin, doramectin can not go through the blood-brain barrier easily , minimal damage to the central nervous system,safe to cattle. The main feature is that the plasma concentration and half-life are higher or twice than Ivermectin.
Precautions
1, doramectin is unstable and decomposes rapidly in the sunlight to be inactivated, its remnants of drugs are toxic to fish and aquatic organisms, so pay attention to water protection.
2, pour-on: bovine application, not rain within 6 hours.
3, use with caution in dogs.
4, the goods will be placed out of reach of children, the operator should not eat or smoking, wash hands after handling.
5, withdrawal period, 35 days of cattle, pigs 24 days.
References
1. Production of doramectin by rational engineering of the avermectin biosynthetic pathway. DOI:10.1016/j.bmcl.2011.04.008
2. Construction of a doramectin producer mutant from an avermectin-overproducing industrial strain of Streptomyces avermitilis.DOI:10.1139/W09-098
3. An alternative derivatization reaction to the determination of doramectin in bovine milk using spectrofluorimetry.DOI:10.1016/j.saa.2012.02.084
4. Doramectin - a potent novel endectocide. DOI:10.1016/0304-4017(93)90218-C
5. Avermectin derivatives, pharmacokinetics, therapeutic and toxic dosages, mechanism of action, and their biological effects (a review). DOI:10.3390/ph13080196
6. Research progress of avermectin: A minireview based on the structural derivatization of avermectin. DOI:10.1016/j.aac.2022.11.001
7. Positive and negative electrospray LC-MS-MS methods for quantitation of the antiparasitic endectocide drugs, abamectin, doramectin, emamectin, eprinomectin, ivermectin, moxidectin and selamectin in milk. DOI:10.1016/J.JCHROMB.2006.11.014
8. https://www.fda.gov/animal-veterinary/cvm-updates/fda-approves-first-generic-doramectin-topical-solution-treatment-certain-parasites-cattle
Properties of Doramectin
| Melting point: | 116-1190C |
| Boiling point: | 967.4±65.0 °C(Predicted) |
| Density | 1.25±0.1 g/cm3(Predicted) |
| vapor pressure | 0Pa at 20℃ |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | methanol: soluble |
| form | solid |
| pka | 12.42±0.70(Predicted) |
| color | white |
Safety information for Doramectin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H362:Reproductive toxicity, effects on or via lactation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P263:Avoid contact during pregnancy/while nursing. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Doramectin
| InChIKey | QLFZZSKTJWDQOS-YDBLARSUSA-N |
Doramectin manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Doramectin 98% (HPLC) CAS 117704-25-3View Details
Doramectin 98% (HPLC) CAS 117704-25-3View Details
117704-25-3 -
 Doramectin CAS 117704-25-3View Details
Doramectin CAS 117704-25-3View Details
117704-25-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1