Domperidone maleate
- CAS NO.:99497-03-7
- Empirical Formula: C26H28ClN5O6
- Molecular Weight: 541.99
- MDL number: MFCD07370074
- EINECS: 801-827-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-12-20 17:31:54

What is Domperidone maleate?
Description
Domperidone maleate is a specific blocker of dopamine receptors. It speeds gastrointestinal peristalsis, causes prolactin release, and is used as antiemetic and tool in the study of dopaminergic mechanisms.
Chemical properties
Domperidone maleate occurs as a white or almost white powder that exhibits polymorphism. It is very slightly soluble in water or alcohol.
The Uses of Domperidone maleate
Domperidone maleate (CAS# 99497-03-7); a form of Domperidone (D531100), a novel peripheral dopamine receptor antagonist that does not cross the blood-brain barrier; anti-emetic. Domperidone(Motilium) is a dopamine blocker and an antidopaminergic reagent.
Indications
Domperidone may be useful for treatment of fescue toxicosis in pregnant mares or as a prokinetic or antiemetic agent in small animals. It has more effect on conditions with delayed gastric emptying than other GI hypomotility conditions. Via its effects on prolactin, domperidone may also be used to stimulate milk production in horses and small animals. Domperidone has been shown to increase plasma ACTH in horses with equine pituitary pars intermedia dysfunction (Equine Cushing’s) and may be useful in helping diagnose this condition.
Preparation
Domperidone was synthesized at Janssen Pharmaceutica in 1974 following their research on antipsychotic drugs. The present invention relates to synthesis of domperidone maleate. O-diaminobenzene is first cyclized and halogenated to form the first benzimidazolone intermediate; 1-carbethoxy-4-amino piperidine is halogenated, reduced, cyclized and hydrolyzed to form the second benzimidazolone intermediate; the two benzimidazolone intermediates are reacted to produce domperidone; and domperidone is finally reacted with maleic aicd to produce domperidone maleate.
https://patents.google.com/patent/CN1810805A/en
Side Effects
Because plasma prolactin levels may be increased, galactorrhea or gynecomastia may result. Injectable products (now withdrawn) have been associated with arrhythmias in human patients with heart disease or hypokalemia. Rarely, somnolence or dystonic reac- tions have occurred in people.
Properties of Domperidone maleate
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | Very slightly soluble in water, sparingly soluble in dimethylformamide, slightly soluble in methanol, very slightly soluble in ethanol (96 per cent). |
| form | Solid |
| color | White to Off-White |
Safety information for Domperidone maleate
Computed Descriptors for Domperidone maleate
Domperidone maleate manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![1-PIPERIDINECARBOXYLIC ACID, 4-[(4-AMINO-5-CHLOROPHENYL)AMINO], ETHYL ESTER](https://img.chemicalbook.in/CAS/20180702/GIF/107618-26-8.gif)
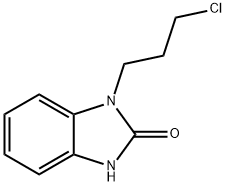
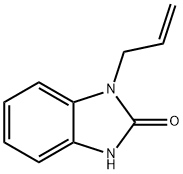
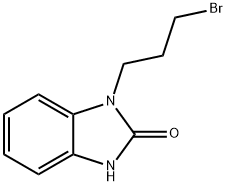
You may like
-
 99497-03-7 98%View Details
99497-03-7 98%View Details
99497-03-7 -
 Domperidone maleate 98%View Details
Domperidone maleate 98%View Details
99497-03-7 -
 Domperidone maleate 99497-03-7 98%View Details
Domperidone maleate 99497-03-7 98%View Details
99497-03-7 -
 99497-03-7 98%View Details
99497-03-7 98%View Details
99497-03-7 -
 Domperidone maleate 99497-03-7 98%View Details
Domperidone maleate 99497-03-7 98%View Details
99497-03-7 -
 99497-03-7 98%View Details
99497-03-7 98%View Details
99497-03-7 -
 Domperidone Maleate 99%View Details
Domperidone Maleate 99%View Details -
 Domperidone maleate 98% (HPLC) CAS 99497-03-7View Details
Domperidone maleate 98% (HPLC) CAS 99497-03-7View Details
99497-03-7
