Domperidone
Synonym(s):4-(5-Chloro-2-oxo-1-benzimidazolinyl)-1-[3-(2-oxobenzimidazolinyl)propyl]piperidine
- CAS NO.:57808-66-9
- Empirical Formula: C22H24ClN5O2
- Molecular Weight: 425.91
- MDL number: MFCD00069256
- EINECS: 260-968-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
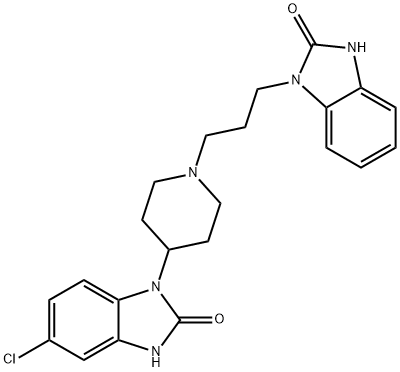
What is Domperidone?
Toxicity
Side effects include galactorrhea, gynecomastia, or menstrual irregularities.
Chemical properties
White or almost white powder.
Originator
Motilium,Cilag,Switz.,1979
The Uses of Domperidone
Domperidone is a medication that enhances peristalsis or contractions of the stomach and intestines. Domperidone is also used to treat nausea and vomiting caused by other medicines used to treat Parkinson's disease.
The Uses of Domperidone
COX2 inhibitor, antiinflammatory, antiarthritic
The Uses of Domperidone
For management of dyspepsia, heartburn, epigastric pain, nausea, and vomiting.
Background
A specific blocker of dopamine receptors. It speeds gastrointestinal peristalsis, causes prolactin release, and is used as antiemetic and tool in the study of dopaminergic mechanisms.
Indications
For management of dyspepsia, heartburn, epigastric pain, nausea, and vomiting.
What are the applications of Application
Domperidone is a peripheral D2DR inhibitor
Definition
ChEBI: 1-[3-(Piperidin-1-yl)propyl]-1,3-dihydro-2H-benzimidazol-2-one in which the 4-position of the piperidine ring is substituted by a 5-chloro-1,3-dihydro-2H-benzimidazol-2-on-1-yl group. A dopamine antagonist, it is used s an antiemetic for the short-term treatment of nausea and vomiting, and to control gastrointestinal effects of dopaminergic drugs given in the management of parkinsonism. The free base is used in oral suspensions, while the maleate salt is used in tablet reparations.
Manufacturing Process
A mixture of 2.3 parts of 1-(3-chloropropyl)-1,3-dihydro-2H-benzimidazol-2- one, 2.5 parts of 5-chloro-1,3-dihydro-l-(4-piperidinyl)-2H-benzimidazol-2- one, 3.2 parts of sodium carbonate, 0.1 part of potassium iodide and 80 parts of 4-methyl-2-pentanone is stirred and refluxed for 24 hours. The reaction mixture is cooled to room temperature and water is added. The undissolved product is filtered off and purified by column chromatography over silica gel using a mixture of trichloromethane and 10% methanol as eluent. The pure fractions are collected and the eluent is evaporated. The residue is crystallized from 4-methyl-2-pentanone. The product is filtered off and recrystallized from a mixture of N,N-dimethylformamide and water, yielding 1.3 parts (30%) of 5- chloro-1-[1-[3-(1,3-dihydro-2-oxo-2H-benzimidazol-1-yl)propyl]-4- piperidinyl]-1,3-dihydro-2H-benzimidazol-2-one; MP 242.5°C.
Side Effects
Common side effects of Domperidone include: headache, dry mouth, diarrhoea or abdominal cramps. They are usually mild and go away on their own. Serious adverse reactions include: seizures or cramps, fast or irregular heartbeat, itching, lumpy rash (hives), or severe allergic reactions.
brand name
Motilium (Janssen);Evixub;Kw 5338;Moperidona;Neta662;R 33812;Tametil;Touristic.
Therapeutic Function
Antiemetic
World Health Organization (WHO)
Domperidone, a peripheral dopaminergic antagonist, was introduced in 1979 for the symptomatic relief of acute nausea and vomiting. The major manufacturer became aware that the injectable formulation was being used in some countries in much higher doses than those recommended to combat nausea and vomiting in cancer patients treated with cytostatic agents. Such use - which was not in conformity with the approved indications - was associated with cardiotoxicity, which in some cases was fatal, and the manufacturer decided to withdraw the injectable dosage form from the market worldwide in January 1985. Suppositories, tablets and a suspension remain available and the manufacturer continues to supply the injection for the treatment of a named patient at the written request of a doctor on the understanding that the appropriate dosage recommendations will be followed.
Biological Activity
Peripheral dopamine D 2 -like receptor antagonist that does not readily cross the blood brain barrier. Displays gastroprokinetic and antiemetic properties; increases the frequency and duration of antral and duodenal contractions and protects from apomorphine-induced emesis (ED 50 values are 0.003 and 0.03 mg/kg for i.v. and oral administration respectively).
Pharmacokinetics
Domperidone is a specific blocker of dopamine receptors. It speeds gastrointestinal peristalsis, causes prolactin release, and is used as antiemetic and tool in the study of dopaminergic mechanisms.
Clinical Use
Acute nausea and vomiting (including that caused by
levodopa and bromocriptine)
Gastro-oesophageal reflux
Dyspepsia
Veterinary Drugs and Treatments
Domperidone may be useful for treatment of fescue toxicosis in
pregnant mares or as a prokinetic or antiemetic agent in small animals.
It has more effect on conditions with delayed gastric emptying
than other GI hypomotility conditions.
Via its effects on prolactin, domperidone may also be used to
stimulate milk production in horses and small animals.
Domperidone has been shown to increase plasma ACTH in
horses with equine pituitary pars intermedia dysfunction (Equine
Cushing’s) and may be useful in helping diagnose this condition.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: possible increased risk of ventricular
arrhythmias with clarithromycin, delamanid and
erythromycin - avoid with clarithromycin and
erythromycin.
Antifungals: possibly increased risk of ventricular
arrhythmias with itraconazole, ketoconazole and
voriconazole - avoid.
Antimalarials: possible increased risk of ventricular
arrhythmias with piperaquine with artenimol -
avoid.
Antivirals: possible increased risk of ventricular
arrhythmias with boceprevir; possible increased risk
of ventricular arrhythmias with ritonavir, saquinavir
and telaprevir - avoid
Apomorphine: possible increased risk of ventricular
arrhythmias.
Cobicistat: possible increased risk of ventricular
arrhythmias - avoid.
Cytotoxics: increased risk of ventricular arrhythmias
with bosutinib and ceritinib - avoid with bosutinib.
Metabolism
Metabolism
Domperidone undergoes extensive first-pass hepatic and
intestinal metabolism. It undergoes rapid and extensive
hepatic metabolism. The main metabolic pathways
are N-dealkylation by cytochrome P450 isoenzyme
CYP3A4, and aromatic hydroxylation by CYP3A4,
CYP1A2, and CYP2E1.
About 30% of an oral dose is excreted in urine within 24
hours, almost entirely as metabolites; the remainder of a
dose is excreted in faeces over several days, about 10% as
unchanged drug.
Storage
Room temperature
Properties of Domperidone
| Melting point: | 240-244°C |
| Density | 1.2904 (rough estimate) |
| refractive index | 1.5400 (estimate) |
| storage temp. | Store at RT |
| solubility | DMSO: >10 mg/mL |
| form | solid |
| pka | pKa 7.90 (Uncertain) |
| color | white |
| Water Solubility | Slightly soluble in water. |
| Merck | 14,3418 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 57808-66-9(CAS DataBase Reference) |
Safety information for Domperidone
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Domperidone
| InChIKey | FGXWKSZFVQUSTL-UHFFFAOYSA-N |
Domperidone manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![1-PIPERIDINECARBOXYLIC ACID, 4-[(4-AMINO-5-CHLOROPHENYL)AMINO], ETHYL ESTER](https://img.chemicalbook.in/CAS/20180702/GIF/107618-26-8.gif)
You may like
-
 Domperidone 99%View Details
Domperidone 99%View Details -
 Domperidone 98% CAS 57808-66-9View Details
Domperidone 98% CAS 57808-66-9View Details
57808-66-9 -
 Domperidone Base API MANUFACTURER INDIAView Details
Domperidone Base API MANUFACTURER INDIAView Details
57808-66-9 -
 Domperidone Api PowderView Details
Domperidone Api PowderView Details
57808-66-9 -
 Domperidone IP PowderView Details
Domperidone IP PowderView Details
57808-66-9 -
 White Domperidone Powder, Medicine GradeView Details
White Domperidone Powder, Medicine GradeView Details
57808-66-9 -
 DOMPERIDONE IP, Non prescription, Treatment: AcidityView Details
DOMPERIDONE IP, Non prescription, Treatment: AcidityView Details
57808-66-9 -
 Medicine Grade Powder DomperidonView Details
Medicine Grade Powder DomperidonView Details
57808-66-9
