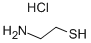
DL-Octopamine hydrochloride
Synonym(s):α-(Aminomethyl)-4-hydroxybenzyl alcohol hydrochloride;(±)-1-(4-Hydroxyphenyl)-2-amino-ethanol hydrochloride
- CAS NO.:770-05-8
- Empirical Formula: C8H12ClNO2
- Molecular Weight: 189.64
- MDL number: MFCD00012881
- EINECS: 212-216-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-27 15:04:49
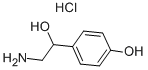
What is DL-Octopamine hydrochloride ?
Chemical properties
DL-Octopamine hydrochloride is a light-yellow crystalline solid and derived from the dried young fruit of Citrus aurantium L., a plant of the Rutaceae family,It is soluble to 10 mg/ml in PBS, 10 mg/ml in EtOH, and 12 mg/ml in DMSO & DMF. Its use is similar to that of Synflorin HCL. It is an adrenergic alpha-receptor stimulant with vasoconstrictive and hypertensive effects.
Originator
Norfen,Morishita, Japan ,1975
The Uses of DL-Octopamine hydrochloride
A biogenic amine that is the phenol analog of Noradrenaline. It is a neurosecretory product found in several vertebrates and invertebrates. Adrenergic.
The Uses of DL-Octopamine hydrochloride
Octopamine HCL has the ability to halt catabolism of proteinaceous tissues. It is very important while losing weight to maximize adipose loss while minimizing the loss of muscle and other lean body tissue. Octopamine Hcl (norepinephrine) is best known as an effective ephedrine alternative, claiming to boost energy while reduce weight in bodybuilding industry for weight loss purpose. In addition, many users believe octopamine hcl is a good nootropic compound that makes our brain easy to focus, enhances motivation as a wakefulness promoter.
What are the applications of Application
Octopamine Hydrochloride is an activator of β3-AR and α2A-AR adrenoceptors
Manufacturing Process
A solution of 33 grams of anhydrous aluminum chloride in 60 grams of nitrobenzene, to which a mixture of 14 grams of phenol and 9.3 grams of hydrochloride of amino-acetonitrile was added, had dry hydrochloric acid gas introduced into it for 3 hours, while stirring and cooling to keep the temperature between 20° and 30°C. The reaction mixture was then poured, with cooling, into 70 cc of water and the deposit obtained was sucked off, washed with acetone and dissolved in 300 cc of water. The solution thus prepared was decolorized with carbon, 50 grams of 30% sodium citrate solution was added to it, and then it was made slightly alkaline with ammonia. Thereupon hydroxy-4'-phenyl-1-amino-2-ethanone crystallized out in the form of leaflets. The yield was 7.7 grams.
The hydrochloride of this base, obtained by evaporation to dryness of a solution of the base in dilute hydrochloric acid and subsequent treatment of the residue with ethyl alcohol and acetone, had a chlorine content of 18.84%, (calculated, 18.90%).
This hydrochloride, on being dissolved in water and hydrogenated with hydrogen and a nickel catalyst, gave a good yield of hydrochloride of hydroxy4'-phenyl-1-amino-2-ethanol melting, after crystallization from a mixture of ethyl alcohol and butanone-2, at from 177° to 179°C with decomposition
Therapeutic Function
Hypertensive
General Description
Octopamine is a biogenic amine, which functions as a neurotransmitter, neuromodulator and neurohormone. It is used to regulate various behaviors, such as learning, memory and aggression. Octopamine is present in mammalian tissues, human urine and invertebrates.
Flammability and Explosibility
Not classified
Biological Activity
Invertebrate biogenic amine neurotransmitter, related to noradrenalin, that is an adrenoceptor agonist. Stimulates lipolysis in mammalian adipocytes via activation of β 3 receptors. Has dual effect on glucose transport in adipocytes: inhibits transport via β 3 receptor activation but stimulates transport when oxidised by MAO. Also activates human α 2A receptors, inhibiting subsequent cAMP production.
Mode of action
Octopamine hydrochloride is an invertebrate biogenic amine neurotransmitter, related to noradrenalin, that is an adrenoceptor (AR) agonist. Octopamine hydrochloride stimulates lipolysis in mammalian adipocytes via activation of β3-AR receptors. Has dual effect on glucose transport in adipocytes: inhibits transport via β3-AR receptor activation but stimulates transport when oxidised by MAO. Also activates human α2A-AR receptors, inhibiting subsequent cAMP production.
Properties of DL-Octopamine hydrochloride
| Melting point: | ~170 °C (dec.)(lit.) |
| Boiling point: | 290℃[at 101 325 Pa] |
| Density | 1.39[at 20℃] |
| vapor pressure | 0.002Pa at 20℃ |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | solid |
| color | white or off-white |
| Water Solubility | soluble |
| Merck | 13,6791 |
| BRN | 3915414 |
| Stability: | Stable. |
| CAS DataBase Reference | 770-05-8(CAS DataBase Reference) |
Safety information for DL-Octopamine hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for DL-Octopamine hydrochloride
New Products
Ethyl 3,3- diethoxypropionate 7-Bromo-2-chloroquinoxaline N-Benzyl-3-hydroxypiperidine 2-(4-bromophenyl)-2-methylpropanoic acid Benzylacetoacetate Radiator Flux Zinc Chloride Solution (All Grades) Phenylazomalononitrile 2-Fluoro-6-iodobenzoic acid 3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 2,3-Difluoro-6-methoxybenzyl Chloride 2-(6-(benzyloxy)-3,4-dihydronaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Ethyl2-oxo-2,3,9,10-tetrahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline-8(7H)-carboxylate Acetyl-meldrum's acid 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid N Ethylmethylamine Ethyl Methanesulfonate N N' DimethylEthylenediamine Lead II BromideRelated products of tetrahydrofuran




You may like
-
 770-05-8 Octopamine hydrochloride 98%View Details
770-05-8 Octopamine hydrochloride 98%View Details
770-05-8 -
 770-05-8 98%View Details
770-05-8 98%View Details
770-05-8 -
 Octopamine HCl 97.00% CAS 770-05-8View Details
Octopamine HCl 97.00% CAS 770-05-8View Details
770-05-8 -
 Dl-octopamine CAS 770-05-8View Details
Dl-octopamine CAS 770-05-8View Details
770-05-8 -
 (±)-Octopamine hydrochloride CAS 770-05-8View Details
(±)-Octopamine hydrochloride CAS 770-05-8View Details
770-05-8 -
 (±)-Octopamine hydrochloride CAS 770-05-8View Details
(±)-Octopamine hydrochloride CAS 770-05-8View Details
770-05-8 -
 5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 -
 4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
79307-93-0
