distrontium diphosphate
Synonym(s):Distrontium diphosphate;Distrontium pyrophosphate;Strontium diphosphate;Strontium metaphosphate oxide
- CAS NO.:13812-81-2
- Empirical Formula: H6O7P2Sr
- Molecular Weight: 267.61
- MDL number: MFCD00799869
- EINECS: 237-461-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
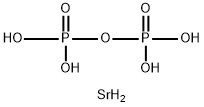
What is distrontium diphosphate?
Description
Strontium pyrophosphate has the molecular formula of Sr2P2O7 and the molecular weight of 349.1833 g/mol.
Its CAS number is 765305-66-6. It may be prepared by
the solid-state reaction of the dibasic orthophosphate,
or alternately by the precipitation of a soluble Mg salt
with aqueous pyrophosphoric acid:
2SrHPO4 + heat ? Sr2P2O7 +H2O
SrCl2 (aq) +H4P2O7 (aq) ? Sr2P2O7 (solid) + 2HCl (aq)
The solution method produces a hemihydrate,
Sr2P2O7·1/2H2O. This salt decomposes at 575°C to
form β-Sr2P2O7. The transition to the high temperature
form, α-Sr2P2O7 takes place at about 760–780°C.
In the Sr2P2O7 system, the unit-cell parameters of
orthorhombic β-Sr2P2O7 are: a = 8.9839?, b = 13.163 ?,
and c = 5.403 ?. The crystallographic data of Sr2P2O7
are reported to contain two types of sites for Sr2+ ions
in the unit cell.
The Uses of distrontium diphosphate
Strontium pyrophosphate has long been used as a matrix for phosphors used in fluorescent lamps in the lamp industry. These phosphors have strong absorption in the near UV region, which is suitable for excitation of ultraviolet light-emitting diodes (UV-LEDs).
The Uses of distrontium diphosphate
Sr2P2O7 is a promising phosphor host material; showing different emission properties upon doping with rare earth and other elements. Example Eu(2+) doped Sr2P2O7 shows blue emission; Sr2P2O7 doped with Bi(2+) is a red phosphor.
Properties of distrontium diphosphate
| Melting point: | >380°C |
| form | powder |
| EPA Substance Registry System | Diphosphoric acid, strontium salt (1:2) (13812-81-2) |
Safety information for distrontium diphosphate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for distrontium diphosphate
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1