CALCIUM PYROPHOSPHATE
Synonym(s):ACP;Dicalcium pyrophosphate
- CAS NO.:7790-76-3
- Empirical Formula: Ca2O7P2
- Molecular Weight: 254.1
- MDL number: MFCD00015983
- EINECS: 232-221-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
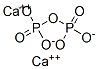
What is CALCIUM PYROPHOSPHATE?
Description
Calcium pyrophosphate has the molecular formula of
Ca2P2O7 and the molecular weight of 254.1378 g/mol. It
has the CAS number of 7790-76-3. It may be prepared by
the solid-state reaction of the dibasic orthophosphate, or
alternately by the precipitation of a soluble Ca salt with
aqueous pyrophosphoric acid:
2CaHPO4 + heat ? Ca2P2O7 +H2O
CaCl2 (aq) +H4P2O7 (aq) ? Ca2P2O7 (solid) + 2HCl (aq)
The solid-state reaction produces an amorphous crystal
at 240–500°C. b-Ca2P2O7 forms >750°C and a-Ca2P2O7
forms in the temperature range of 1140 to 1350°C. The
free energy of formation, △Gf=-3132 kcal/mol.
The product of the aqueous method is the dihydrate,
Ca2P2O7·2H2O. It is a white powder and has the CAS
number of 7790-76-3. It is insoluble in water but soluble
in dilute acids. The anhydrate decomposes at 1353°C to
form a metaphosphate:
Ca2P2O7 + heat ? Ca(PO3)2 + CaO
Calcium pyrophosphate is dimorphic. The phase
transformation and sintering behaviors of Ca2P2O7 with
different phase composition has been investigated by
using X-ray powder diffraction (XRD), dilatometery and
SEM techniques. It was found that although α-Ca2P2O7
(high-temperature form) could be maintained during its
cooling process, it was metastable and retransformed
into β-Ca2P2O7 (low-temperature form) at about 950 °C
during its reheating process.
Chemical properties
white powder
The Uses of CALCIUM PYROPHOSPHATE
Calcium Pyrophosphate is a nutrient and dietary supplement that exists as a white odorless powder, insoluble in water. it is used in dental impression materials and as a buffer.
The Uses of CALCIUM PYROPHOSPHATE
Calcium phosphate is used in the production of phosphoric acid and fertilizers. It is used in baking as the acid in a leavening agent. It is also used in cheese products. This compound has also been used in gene transfection of cells. It is also being used in the development of contrast agents.
Definition
ChEBI: Calcium diphosphate is a calcium phosphate. It contains a diphosphate(4-).
Flammability and Explosibility
Non flammable
Properties of CALCIUM PYROPHOSPHATE
| Melting point: | 1230 °C |
| Density | 3.09 g/mL at 25 °C(lit.) |
| solubility | H2O: insoluble(lit.) |
| form | Powder |
| Specific Gravity | 3.09 |
| color | White |
| Odor | at 100.00?%. odorless |
| Water Solubility | Soluble in hydrochloric acid and nitric acid. Insoluble in water. |
| CAS DataBase Reference | 7790-76-3(CAS DataBase Reference) |
| EPA Substance Registry System | Diphosphoric acid, calcium salt (1:2) (7790-76-3) |
Safety information for CALCIUM PYROPHOSPHATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for CALCIUM PYROPHOSPHATE
CALCIUM PYROPHOSPHATE manufacturer
ShanPar Industries Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 6-Bromo-3-iodo-1-methyl-1H-indazole 4-Ethylbenzylamine N-(5-Amino-2-methylphenyl)acetamide 2-(BOC-Amino)4-picoline 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazoleRelated products of tetrahydrofuran
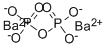
You may like
-
 Calcium pyrophosphate 98%View Details
Calcium pyrophosphate 98%View Details -
 35405-51-7 / 7790-76-3 99%View Details
35405-51-7 / 7790-76-3 99%View Details
35405-51-7 / 7790-76-3 -
 Calcium pyrophosphate 98%View Details
Calcium pyrophosphate 98%View Details
35405-51-7 / 7790-76-3 -
 Calcium phosphate (pyro) CAS 7790-76-3View Details
Calcium phosphate (pyro) CAS 7790-76-3View Details
7790-76-3 -
 Calcium pyrophosphate, puriss, ≥98% CAS 7790-76-3View Details
Calcium pyrophosphate, puriss, ≥98% CAS 7790-76-3View Details
7790-76-3 -
 Calcium phosphate, amorphous CAS 7790-76-3View Details
Calcium phosphate, amorphous CAS 7790-76-3View Details
7790-76-3 -
 100-71-0 99%View Details
100-71-0 99%View Details
100-71-0 -
 1446013-08-6 98%View Details
1446013-08-6 98%View Details
1446013-08-6