DIMETHYLKETENE METHYL TRIMETHYLSILYL ACETAL
Synonym(s):(1-Methoxy-2-methyl-1-propenyloxy)trimethylsilane;1-Methoxy-2-methyl-1-(trimethylsiloxy)propene;MTDA
- CAS NO.:31469-15-5
- Empirical Formula: C8H18O2Si
- Molecular Weight: 174.31
- MDL number: MFCD00010232
- EINECS: 629-515-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-21 22:12:10
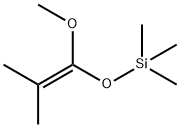
What is DIMETHYLKETENE METHYL TRIMETHYLSILYL ACETAL?
Chemical properties
Clear colorless liquid
Physical properties
bp 35 °C/15 mmHg; d 0.858 g cm?3.
The Uses of DIMETHYLKETENE METHYL TRIMETHYLSILYL ACETAL
1-Methoxy-2-methyl-1-(trimethylsilyloxy) propene is widely used as functional equivalent of enolate of methyl isobutyrate; ester enolate surrogate in electrophilic reactions including alkylation, aldol reaction, Michael reaction, initiator for group transfer polymerization of acrylates, nitroarylation, oxidation, dimerization, and cycloadditions.
The Uses of DIMETHYLKETENE METHYL TRIMETHYLSILYL ACETAL
1-Methoxy-2-methyl-1-(trimethylsiloxy)propene is used in the synthesis of chiral β-lactams by reacting with (S)-alkylidene(1-arylethyl)amines in the presence of titanium tetrachloride. It acts as a catalyst or initiator in the group-transfer polymerization. It is also used as a versatile reagent in conjugate addition4 and aldol reactions.
What are the applications of Application
Methyl trimethylsilyl dimethylketene acetal is Methyl trimethylsilyl dimethylketene acetal has been used: ? in the synthesis of chiral β-lactams by reacting with (S)-alkylidene(1-arylethyl)amines in the presence of titanium tetrachloride ? as a catalyst or initiator in the group-transfer polymerization ? as a versatile reagent in conjugate addition and aldol reactions.
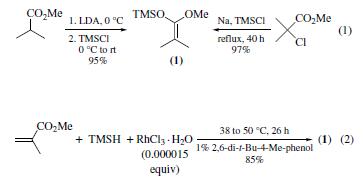
Preparation
The title compound is a prototypical ketene silyl acetal (KSA) that can been prepared by either of the two most commonly employed methods: (a) deprotonation of the |á-hydrogen of an ester followed by silylation (1),16 and (b) metal-catalyzed hydrosilylation of |á,|?-unsaturated esters(2).
Purification Methods
Add Et2O, wash with cold H2O, dry (Na2SO4), filter, evaporate Et2O, and distil the oily residue in a vacuum. [Ainsworth et al. J Organometal Chem 46 59 1972.]
Properties of DIMETHYLKETENE METHYL TRIMETHYLSILYL ACETAL
| Boiling point: | 35 °C15 mm Hg(lit.) |
| Density | 0.858 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 58 °F |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | freely sol organic solvents. |
| form | clear liquid |
| color | Colorless to Almost colorless |
| Specific Gravity | 0.858 |
| Water Solubility | Hydrolyzes in water. |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 1362893 |
| Stability: | Moisture and Acid Sensitive |
| CAS DataBase Reference | 31469-15-5(CAS DataBase Reference) |
| EPA Substance Registry System | Silane, [(1-methoxy-2-methyl-1-propenyl)oxy]trimethyl- (31469-15-5) |
Safety information for DIMETHYLKETENE METHYL TRIMETHYLSILYL ACETAL
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for DIMETHYLKETENE METHYL TRIMETHYLSILYL ACETAL
New Products
Bromine 99.5% AR (4 x 500ml) Fehling's Solution No. B Amino Acid Kit of 23 items set Ammonium Molybdate Reagent Solution Beam's Reagent Solution Ehrlich's Reagent For detection of urobillinogen Sodium Amino Salicylate Dihydrate (PAS Sodium) IP/BP/USP/EP 1,2,3,4-Tetrahydrocarbazol-4-one 4-Hydroxy Carbazole Amino Salicylic Acid. U.S.P. 2 – Methoxy – 5- Sulfamoyl Benzoic acid Acetone Isobutryl oxime ester Curcuma aromatica Oil Curry leaf Extract Terminalia bellirica Extract Aloe vera extract 200x Withania somnifera (Ashwagandha Extract) Citrus bioflavonoids Extract Ethyl 3-(Pyridin-2-Ylamino)Propanoate Bilastine -IP/BP/ Cypermethric Acid Chloride 5-Nitrosalicylaldehyde 5-(Difluoromethoxy)-2-Mercapto-1H-Benzimidazole- IP/BP/ Methyl Di Chloride (Mdc)Related products of tetrahydrofuran

You may like
-
 1-Methoxy-2-methyl-1-(trimethylsiloxy)propene CAS 31469-15-5View Details
1-Methoxy-2-methyl-1-(trimethylsiloxy)propene CAS 31469-15-5View Details
31469-15-5 -
 Dimethylketene Methyl Trimethylsilyl Acetal CAS 31469-15-5View Details
Dimethylketene Methyl Trimethylsilyl Acetal CAS 31469-15-5View Details
31469-15-5 -
 Dimethylketene methyl trimethylsilyl acetal 96% CAS 31469-15-5View Details
Dimethylketene methyl trimethylsilyl acetal 96% CAS 31469-15-5View Details
31469-15-5 -
 Methyl trimethylsilyl dimethylketene acetal CAS 31469-15-5View Details
Methyl trimethylsilyl dimethylketene acetal CAS 31469-15-5View Details
31469-15-5 -
 7726-95-6 Bromine 99.5% AR (4 x 500ml) 99%View Details
7726-95-6 Bromine 99.5% AR (4 x 500ml) 99%View Details
7726-95-6 -
 Formamide 99%View Details
Formamide 99%View Details
75-12-7 -
 85-81-4 6-Methoxy-8-Nitroquinoline 99%View Details
85-81-4 6-Methoxy-8-Nitroquinoline 99%View Details
85-81-4 -
![3-Bromo-4,5-Dihydro-1H-Benzo[B]Azepin-2(3H)-One 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 3-Bromo-4,5-Dihydro-1H-Benzo[B]Azepin-2(3H)-One 99%View Details
3-Bromo-4,5-Dihydro-1H-Benzo[B]Azepin-2(3H)-One 99%View Details
86499-96-9