Diisopropyl peroxydicarbonate
- CAS NO.:105-64-6
- Empirical Formula: C8H14O6
- Molecular Weight: 206.19
- MDL number: MFCD01708854
- EINECS: 203-317-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 15:16:10
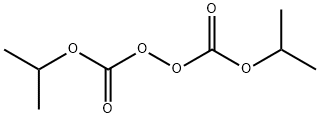
What is Diisopropyl peroxydicarbonate?
Chemical properties
White crystalline solid with a mild characteristicodor; mp 8–10°C (46.4–50°F); density1.080 at 15°C (59°F); slightly soluble inwater, 0.04% at 25°C (77°F), soluble in ether,chloroform, benzene, hexane, and isooctane.
The Uses of Diisopropyl peroxydicarbonate
It is used as a catalyst to initiate polymerizationreactions to produce polymers fromethylene, styrene, vinyl acetate, vinyl chloride,and vinyl esters. Polymers of increasedlinearity and better properties can be obtainedby optimizing its concentration, temperature,and pressure conditions.
Synthesis Reference(s)
Journal of the American Chemical Society, 72, p. 1254, 1950 DOI: 10.1021/ja01159a052
General Description
A white solid (shipped packed in Dry Ice to stabilize) with a sharp unpleasant odor. Insoluble in sater and sinks in water. Used as polymerization catalyst.
Air & Water Reactions
Highly flammable. Insoluble in water. Spontaneous decomposition at room temperature releases flammable toxic products.
Reactivity Profile
ISOPROPYL PEROXYDICARBONATE decomposes violently or explosively at temperatures 0-10°C owing to self- accelerating exothermic decomposition; Several explosions were due to shock, heat, or friction; amines and certain metals can cause accelerated decomposition [Bretherick 1979 p. 156]. Spontaneous decomposition possible at room temperature to release flammable and corrosive products (presence of stabilizer reduces this possibility). A strong oxidizing agent. May ignite organic compounds on contact, hence a fire risk. Strongly reduced material such as sulfides, nitrides, and hydrides can react explosively. Reacts at least to produce heat when mixed with members of most chemical classes. These reactions often generate gases (toxic and nontoxic). Subject to decomposition, buildup of heat and even an explosion if contaminated with a catalyst (often a transition metal such as cobalt, iron, manganese, nickel, or vanadium or a salt of a transition metal).
Health Hazard
Inhalation overexposure unlikely, but prolonged exposure may cause lung edema. Contact with eyes may cause irritation. Solutions are severe primary skin irritants.
Health Hazard
Diisopropyl peroxydicarbonate is a moderateskin irritant. The irritation can be severe onrabbit skin. Eye contact can cause conjunctivitisand corneal ulcerations, which, however,are fully recoverable in 1–2 weeks. Itstoxicity is very low. There is no report of itsill effect on humans. It may cause dermatitison sensitive skin.
LD50 value, oral (rats): 21,410 mg/kg.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: May decompose with the formation of oxygen when in contact with metals; Stability During Transport: Unstable at temperatures above 0°F with the formation of oxygen gas; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Moderately toxic by ingestion and skin contact. A severe eye irritant. Very dangerous fire hazard. Dangerously unstable above 10°C. An impactand heat-sensitive explosive. Solutions may spontaneously explode (the hazard increases with concentration). Storage in sealed containers map be dangerous. Explodes on contact with amines or potassium iodide. May explode on contact with organic matter. When heated to decomposition it emits acrid smoke and fumes. See also PEROXIDES, ORGANIC
Waste Disposal
Larger quantities can be disposed of by scatteringin a disposal area where it melts andgradually decomposes. Small quantities aredestroyed by adding slowly to 5% alcoholicKOH. Solutions in small amount may be disposedof in porous sandy soils.
Properties of Diisopropyl peroxydicarbonate
| Melting point: | 12°C |
| Boiling point: | 265.04°C (rough estimate) |
| Density | 1.080 |
| vapor pressure | 17.1hPa at 20℃ |
| refractive index | 1.4034 |
| Odor | Disagreeable; pungent. |
| Water Solubility | 30.5mg/L at 20℃ |
| Exposure limits | No exposure limit is set. Because of low
toxicity and vapor pressure, the health hazard
from its exposure does not arise. |
| Stability: | Shock-sensitive. Highly reactive. Heat sensitive. May explode or ignite in contact with organic material. |
| EPA Substance Registry System | Peroxydicarbonic acid, C,C'-bis(1-methylethyl) ester (105-64-6) |
Safety information for Diisopropyl peroxydicarbonate
Computed Descriptors for Diisopropyl peroxydicarbonate
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4