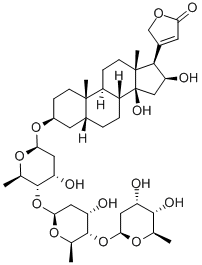
Digitoxin
Synonym(s):;Digitoxin;Digitoxoside;Lanatoxin
- CAS NO.:71-63-6
- Empirical Formula: C41H64O13
- Molecular Weight: 764.94
- MDL number: MFCD00003686
- EINECS: 200-760-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-20 16:56:16
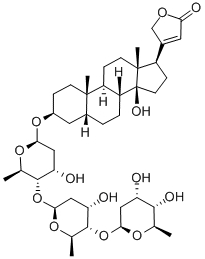
What is Digitoxin?
Toxicity
Digitoxin exerts similar toxic effects to digoxin including anorexia, nausea, vomiting, diarrhoea, confusion, visual disturbances, and cardiac arrhythmias.
Description
Digitoxin is the most powerful and reliable of the glucosides which have been extracted from digitalis leave. It is a cardiac glycoside (CG). It is a cardiotonic drug, which exhibits cardiac and anti-cancer properties. Digitoxin inhibits nuclear factor kappa B (NF-κB) signaling. It is used to treat congestive heart failure and cardiac arrhythmia. Digitoxin prevents microtubule formation.
Description
Digitoxin is a cardiac glycoside that has been found in Digitalis and has diverse biological activities. It inhibits human recombinant α1β1, α2β2, and α3β1 subunit-containing Na+/K+-ATPases with Ki values of 250, 63, and 136 nM, respectively. Digitoxin inhibits the human-ether-a-go-go (hERG) potassium channel, also known as Kv11.1, in HEK293 cells expressing hERG (IC50 = 11.1 nM). It enhances developed tension and contractile force in electrically stimulated isolated guinea pig left atrial muscle when used at concentrations of 0.2 and 0.4 μM, respectively. Dietary administration of digitoxin (~1 mg/kg per day) attenuates congestive heart failure and reduces myocardial hypertrophy in a rat model of myocardial infarction induced by coronary artery ligation. Digitoxin is also cytotoxic to a panel of 10 human cancer cell lines, including myeloma, lymphoma, and leukemia cancer cells, with IC50 values ranging from 12 to 76 nM. Formulations containing digitoxin have previously been used in the treatment of congestive heart failure and cardiac arrhythmias.
Chemical properties
White Solid
Originator
Crystodigin,Lilly
The Uses of Digitoxin
Digitoxin has been used as a labeled drugs for binding sites on human serum albumin (HSA). It has also been used to test its anti-transmissible gastroenteritis virus (TGEV) activity.
The Uses of Digitoxin
Digitoxin is used for chronic cardiac insufficiency, tachyarrhythmia form of atrial fibrillation, paroxysmal ciliary arrhythmia, and paroxysmal supraventricular tachycaria.
Indications
For the treatment and management of congestive cardiac insufficiency, arrhythmias and heart failure.
Background
A cardiac glycoside sometimes used in place of digoxin. It has a longer half-life than digoxin; toxic effects, which are similar to those of digoxin, are longer lasting. (From Martindale, The Extra Pharmacopoeia, 30th ed, p665)
What are the applications of Application
Digitoxin is a cardiac glycoside
Indications
Digitoxin (Dig) is an FDA approved drug for the treatment of cardiac disease.
Digitoxin is used for the treatment of heart failure, especially in people with impaired kidney function. It is also used to treat certain kinds of heart arrhythmia, such as atrial fibrillation.
Digoxin is used to treat heart failure and abnormal heart rhythms (arrhythmias). It also helps the heart work better and control heart rate. Digoxin may be used after a heart attack. This medication comes in various forms: tablet, capsule, or pediatric elixir (liquid). It is available under the brand names Lanoxin, Cardoxin, Digitek, Digox, and Lanoxicaps.
Definition
ChEBI: Digitoxin is a cardenolide glycoside in which the 3beta-hydroxy group of digitoxigenin carries a 2,6-dideoxy-beta-D-ribo-hexopyranosyl-(1->4)-2,6-dideoxy-beta-D-ribo-hexopyranosyl-(1->4)-2,6-dideoxy-beta-D-ribo-hexopyranosyl trisaccharide chain. It has a role as an EC 3.6.3.9 (Na(+)/K(+)-transporting ATPase) inhibitor. It is functionally related to a digitoxigenin. It is a conjugate acid of a digitoxin(1-).
Manufacturing Process
1000 g of Digitalis purpurea leaves were moistened thoroughly with a
menstruum consisting of 60% ethyl alcohol and 40% water and were packed
in a percolator with enough of the menstruum to leave a stratum above the
drug. After maceration overnight, the drug was percolated with about 7 liters of the 60%-alcohol menstruum and about 5 liters of percolate or extract were
collected. 400 g of solid lead acetate were added to the percolate and the
mixture was stirred until all the lead acetate had dissolved. After standing for
at least one hour, the copious light green precipitate was centrifuged off and
washed successively with 1000 ml and 500 ml portions of 60% alcohol. The
washings were combined with the filtrate from the centrifuge and most of the
excess lead acetate removed by treatment with a saturated solution of sodium
carbonate monohydrate. The resulting lead carbonate was filtered off, washed
with two 200 ml portions of 60% ethyl alcohol, and the washings combined
with the filtrate. Hydrogen sulfide was then passed through the combined
liquids until no more lead sulfide precipitated. The filtrate and washings
resulting from filtering off the lead sulfide were concentrated in vacuo at or
below 40°C to a volume of 2000 ml and saturated with a salt, such as sodium
chloride, to facilitate subsequent extraction with a water-immiscible organic
solvent. The mixture was extracted five times with 600 ml of a solvent
consisting of two volumes of chloroform and three volumes of amyl ether. The
chloroform-amyl ether solution is extracted with about four 400 ml portions of
a 10% solution of sodium carbonate monohydrate to remove any gitalin that
may have carried through in the process and vegetative extractive material.
After drying over anhydrous sodium sulfate and filtering, the chloroform-amyl
ether solution was concentrated in vacuo at 75°-85°C to a volume of about 25
ml. After cooling to room temperature, the concentrate was mixed with about
four volumes of petroleum ether and allowed to stand for about one hour at
room temperature. The dark colored, amorphous precipitate was filtered and
washed with petroleum ether to ensure that all fat had been removed. The
precipitate was dissolved in 100 ml of dilute alcohol (1:1) and the slight
precipitate remaining after thorough agitation was filtered off. The filtrate was
made slightly alkaline with 10% ammonia water and 10 g of solid lead acetate
were dissolved therein with agitation. The light brown precipitate, which
formed, was centrifuged off and washed with two 50 ml portions of dilute
alcohol. Excess lead acetate was removed by passage of hydrogen sulfide
through the solution until no more lead sulfide precipitated. The filtrate and
washings resulting from removal of the lead sulfide was concentrated below
40°C. After making slightly alkaline with ammonia water, the concentrate was
extracted with three 50 ml portions of chloroform. The chloroform extract of
digitoxin was dried over anhydrous sodium sulfate. After filtering and washing
the filter with dry chloroform, the chloroform extract was heated on a water
bath to remove the chloroform and the residue was dissolved in 20ml of hot
alcohol at about 60°C., and diluted with hot distilled water at 60°C to an
alcohol concentration of 30%. Upon standing overnight, the digitoxin settled
out as a yellowish orange, mostly amorphous solid together with some needle
and rosette crystals.
The digitoxin was filtered off, and dried in a vacuum desiccator over calcium
chloride and then was dissolved In 10 cc. of dry chloroform after which 15 ml
of dry amyl ether was added, followed by 100 ml of petroleum ether. After
standing one hour, the precipitate was filtered off, washed with petroleum
ether, and dried in a vacuum desiccator until all traces of amyl ether were
removed. One 1ml of alcohol for each 25 milligrams of material was added to
the dried precipitate and the mixture was heated on a water-bath at 60°C
until the precipitate had completely dissolved, after which hot distilled water
at 60°C was added to produce an alcohol concentration of 40%. Upon
standing overnight at room temperature the digitoxin came down as almost
completely white crystals. Upon recrystallizing a second time from 40%
alcohol, completely white crystals of digitoxin were obtained. On the basis of the digitalis cat assay, the digitoxin was completely pure and is a prompt and
powerful heart tonic in doses of 25 mg to 1 mg. The crystalline digitoxin is
also substantially stable and may be relied upon by the physician to furnish a
uniform degree of activity of the same kind insofar as the digitoxin is
concerned.
brand name
Crystodigin (Lilly) .
Therapeutic Function
Cardiotonic, Topical venotonic
General Description
Odorless white or pale buff microcrystalline powder. Used as a cardiotonic drug.
Health Hazard
Material is bioactive and capable of causing cardiac arrythmias and electrolyte imbalances that may be fatal. Death is due to ventricular fibrillation or cardiac standstill. Material has a high toxicity hazard rating; it may cause death or permanent injury after a very short exposure. It is classified as super toxic; an estimated single lethal dose is 3-10 mg.
Fire Hazard
When heated to decomposition, DIGITOXIN emits acrid smoke and irritating fumes.
Pharmacokinetics
Digitoxin is a cardiac glycoside sometimes used in place of DIGOXIN. It has a longer half-life than digoxin; toxic effects, which are similar to those of digoxin, are longer lasting (From Martindale, The Extra Pharmacopoeia, 30th ed, p665). It is eliminated hepatically making it useful in patients with poor or erratic kidney function, although it is now rarely used in practice. Digitoxin lacks the strength of evidence that digoxin has in the management of heart failure.
Safety Profile
A deadly poison by most routes. Human systemic effects: arrhythmias, cardiomyopathy, EKG changes, nausea or vomiting, paresthesia, pulse rate increase, thrombocytopenia. Human reproductive effects by ingestion: reduced viability of newborn. An eye irritant. When heated to decomposition it emits acrid smoke and irritating fumes. See also DIGITALIS.
Potential Exposure
NaturalProduct; Reproductive Effector; Primary Irritant. This material is used as a cardiotonic drug. Digitoxin is the most toxiccomponent of digitalis.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Metabolism
Hepatic.
Storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with digitoxinyou should be trained on its proper handling and storage.Store in tightly closed containers in a refrigerator.
Shipping
This compound can be classed under Medicine,solid, toxic, n.o.s. It requires a shipping label of“POISONOUS/TOXIC MATERIALS.” It falls in HazardClass 6.1 and Packing Group III.
Purification Methods
Digitoxin crystallises from MeOH, aqueous EtOH with 0.5 to 1 H2O and from H2O as the dihydrate. It also crystallises from CHCl3/Et2O as anhydrous crystals. The hydrate dehydrates at 120o/vacuum. Its solubility is 2.5% in CHCl3, 1.7% in EtOH, 0.25% in EtOAc, and 0.001% in H2O; and has E1cm 202.5 at 219-220nm (50% EtOH). [Stoll et al. Helv Chim Acta 37 1134 1954, Demoen & Janssen J Am Pharm Assoc 42 635 1953, Beilstein 18 IV 1478.]
Mode of action
Digitoxin is a purified cardiac glycoside similar in structure and function to Digoxin. Unlike Digoxin, Digitoxin is eliminated from the body via the liver and not the kidneys. Both drugs are used to treat various heart conditions. Cardiac glycosides bind to a site on the extracellular aspect of the a-subunit of the Na+/K+ ATPase pump in the membranes of heart cells (myocytes). This causes an increase in the level of sodium ions in the myocytes, which then leads to a rise in the level of calcium ions. Digitoxin inhibits the sodium-potassium ATPase in heart muscle cells, resulting in increased force of contractions (positive inotropic), reduced speed of electric conduction (negative dromotropic), increased excitability (positive bathmotropic), and reduced frequency of heartbeat (negative chronotropic).
Properties of Digitoxin
| Melting point: | 240 °C (dec.)(lit.) |
| Boiling point: | 654.47°C (rough estimate) |
| alpha | D20 +4.8° (c = 1.2 in dioxane) |
| Density | 1.0971 (rough estimate) |
| refractive index | 17 ° (C=2, CHCl3) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | chloroform: soluble |
| form | powder |
| pka | 13.50±0.70(Predicted) |
| color | White |
| Water Solubility | 3.9mg/L(25 ºC) |
| Merck | 14,3163 |
| BRN | 76678 |
| Stability: | Hygroscopic |
| EPA Substance Registry System | Digitoxin (71-63-6) |
Safety information for Digitoxin
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H331:Acute toxicity,inhalation H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P314:Get medical advice/attention if you feel unwell. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Digitoxin
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Digitoxin CAS 71-63-6View Details
Digitoxin CAS 71-63-6View Details
71-63-6 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
