DIETHYLCARBAMAZINE
- CAS NO.:90-89-1
- Empirical Formula: C10H21N3O
- Molecular Weight: 199.29
- MDL number: MFCD00023288
- EINECS: 202-023-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
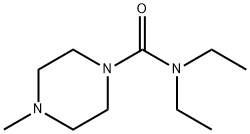
What is DIETHYLCARBAMAZINE?
Absorption
Readily absorbed following oral administration.
Toxicity
Oral LD50 in rat and mouse is 1400 mg/kg and 660 mg/kg, respectively.
Description
Discovered in the 1940s, diethylcarbamazine (DEC) has proven to be especially effective as a filaricidal agent. The incidence of filariasis among American troops during World War II necessitated a search for drugs with an antifalarial spectrum of activity. The once-popular piperazine also was discovered during these initial screenings. Although chemically similar, the activity again helminths is quite different. Piperazine is active against nematodes, whereas DEC is active against falaria and microfalaria.
Originator
Hetrazan,Lederle,US,1949
Indications
Used for the treatment of certain filarial diseases, including tropical pulmonary eosinophilia, loiasis, and lymphatic filariasis caused by infection with Wuchereria bancrofti, Brugia malayi, or Brugia timori.
Background
An anthelmintic used primarily as the citrate in the treatment of filariasis, particularly infestations with Wucheria bancrofti or Loa loa.
Indications
Diethylcarbamazine citrate (Hetrazan) is active against
several microfilaria and adult filarial worms. It interferes
with the metabolism of arachidonic acid and
blocks the production of prostaglandins, resulting in
capillary vasoconstriction and impairment of the passage
of the microfilaria. Diethylcarbamazine also increases
the adherence of microfilariae to the vascular
wall, platelets, and granulocytes.
Diethylcarbamazine is absorbed from the gastrointestinal
tract, and peak blood levels are obtained in 4
hours; the drug disappears from the blood within 48
hours. The intact drug and its metabolites are excreted
in the urine.
Diethylcarbamazine is the drug of choice for certain
filarial infections, such as Wuchereria bancrofti, Brugia
malayi and Loa loa. Since diethylcarbamazine is not
universally active against filarial infections, a specific diagnosis
based on blood smears, biopsy samples, and a
geographic history is important. Dosage should be adjusted
in patients with renal impairment.
Caution is necessary when using this agent, particularly
when treating onchocerciasis.The sudden death of
the microfilariae can produce mild to severe reactions
within hours of drug administration. These are manifested
by fever, lymphadenopathy, cutaneous swelling,
leukocytosis, and intensification of any preexisting
eosinophilia, edema, rashes, tachycardia, and headache.
If microfilariae are present in the eye, further ocular
damage may result. Other side effects are relatively
mild and range from malaise, headache, and arthralgias
to gastrointestinal symptoms.
Definition
ChEBI: Diethylcarbamazine is a N-methylpiperazine and a N-carbamoylpiperazine.
Manufacturing Process
To 50 cc of water was added 18 grams of 1-methylpiperazine dihydrochloride
and 8.34 grams of sodium hydroxide. When solution had been effected the
beaker was cooled to 10°C and with stirring, 4.17 grams of sodium hydroxide
dissolved in 15 cc of water and 14 grams of diethyl carbamyl chloride were
added simultaneously. When all had been added, the solution was extracted 3
times with ether which was then dried and filtered. The ether solution was
saturated with dry hydrogen chloride. A yellow gum appeared which on
trituration gave a white, hygroscopic solid which was filtered and dried in a
drying pistol. The N,N-diethyl-4-methyl-1-piperazine-carboxamide
hydrochloride had a melting point of 150°-155°C.
If the compound itself is desired, the salt is dissolved in water and the
solution saturated with a mild alkali such as potassium carbonate. The product
is then extracted with chloroform, dried, and after removal of the chloroform,
distilled.
Therapeutic Function
Anthelmintic
Synthesis Reference(s)
The Journal of Organic Chemistry, 13, p. 144, 1948 DOI: 10.1021/jo01159a019
Diethylcarbamazine
Antimicrobial activity
Useful activity is restricted to filarial worms. It is adulticidal and microfilaricidal against Loa loa. Against Wuchereria bancrofti and Brugia malayi it is predominantly microfilaricidal, but slowly kills adult worms. It kills microfilariae, but not adults, of Onchocerca volvulus.
Pharmaceutical Applications
A carbamyl derivative of piperazine formulated as the citrate. It is readily soluble in water and slightly hygroscopic.
Mechanism of action
Although studied extensively, the mechanism of action of DEC remains unknown. Diethylcarbamazine appears to be the active form of the drug, with a very rapid onset of action (within minutes), but of interest is the fact that the drug is inactive in vitro, suggesting that activation of a cellular component is essential to the filaricidal action. Three mechanisms have been suggested. The first is involvement of blood platelets triggered by the action of filarial excretory antigens. A complex reaction is thought to occur between the drug, the antigen, and platelets. Although these authors were unable to show a direct action of the drug on the microfalaria, a more recent study showed that DEC produced morphological damage to the microfalaria. The damage consisted of the loss of the cellular sheath, exposing antigenic determinants to immune defense mechanisms. Severe damage then occurred to microfalaria organelles, leading to death. The second is inhibition of microtubule polymerization and disruption of preformed microtubules. The third is interference with arachidonic acid metabolism. Diethylcarbamazine is known to have anti-inflammatory action, which appears to involve blockage at cyclooxygenase and leukotriene A4 synthase (leukotriene synthesis). This action appears to alter vascular and cellular adhesiveness and cell activation. This latter action would suggest a possible relationship between the first and third mechanism.
Pharmacokinetics
Diethylcarbamazine is an anthelmintic drug that does not resemble other antiparasitic compounds. It is a synthetic organic compound which is highly specific for several parasites and does not contain any toxic metallic elements.
Pharmacokinetics
Oral absorption: >90%
Cmax 200 mg: 1.5–2 mg/L after 2 h
Plasma half-life: c. 6–12 h
Volume of distribution: 107–371 L
Plasma protein binding: Very low
Like piperazine (to which it is related), diethylcarbamazine
is rapidly and completely absorbed. About half the dose is
excreted unchanged in the urine; the rest is metabolized and
eliminated by renal and extrarenal routes.
Clinical Use
Filariasis
It has also been used for visceral larva migrans, but experience
is limited and there is little evidence of its efficacy.
Side Effects
In uninfected people, diethylcarbamazine has virtually no side effects, but in those with various forms of filariasis it has unpleasant effects primarily due to the death of bloodor skin-dwelling microfilariae. Severe reactions (‘Mazzotti reactions’), most frequently of the skin, occur in patients with onchocerciasis and may also be systemic with fever, headache, prostration, nausea, joint and muscle pain, vertigo, tachycardia, cough and respiratory distress, hypotension and ocular signs. In patients with L. loa who harbor very large numbers of microfilariae in their blood, neurological problems may be very severe. Cardiological damage has also been reported. In patients with W. bancrofti and B. malayi high fever occurs in the first few days after treatment. Reversible proteinuria may occur.
Safety Profile
Poison by intraperitoneal route. Human systemic effects by ingestion: allergc dermatitis. An experimental teratogen. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx and HCl. An additive permitted in the food and drinking water of animals and/or for the treatment of food-producing animals.
Synthesis
Diethylcarbamazine, N, N-diethyl-4-methyl-1-piperazincarboxamide (38.1.13), is made by acylating 1-methylpiperazine with diethylcarbamoylchloride.
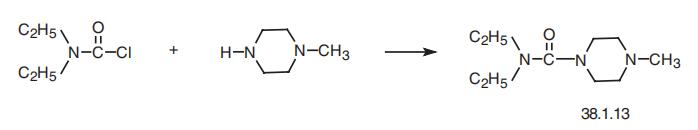
Metabolism
Partially metabolized to diethylcarbamazine N-oxide.
Metabolism
The metabolism of DEC leads to the compounds shown in Figure 39.18 plus trace amounts of
methylpiperazine and piperazine. Nearly all of the metabolites appear in the urine. As much as 10
to 20% of the drug is excreted unchanged. As indicated by the rapid action of the drug, it would
appear that none of the metabolites are involved in the therapeutic action of DEC.
Properties of DIETHYLCARBAMAZINE
| Melting point: | 65-67℃ |
| Boiling point: | 127-129℃ (10 Torr) |
| Density | 1.0173 (rough estimate) |
| refractive index | 1.4930 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| pka | pKa 7.7 (Uncertain) |
| EPA Substance Registry System | Diethylcarbamazine (90-89-1) |
Safety information for DIETHYLCARBAMAZINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. P501:Dispose of contents/container to..… |
Computed Descriptors for DIETHYLCARBAMAZINE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![4-((4-[(5-CHLORO-1-BENZOFURAN-2-YL)METHYL]PIPERAZIN-1-YL)CARBONYL)MORPHOLINE](https://img.chemicalbook.in/StructureFile/ChemBookStructure21/GIF/CB7825183.gif)

![5-(4-FLUOROPHENYL)-8-[4-(MORPHOLIN-4-YLCARBONYL)PIPERAZIN-1-YL]-1,7-NAPHTHYRIDINE](https://img.chemicalbook.in/StructureFile/ChemBookStructure21/GIF/CB2841247.gif)
![8-[4-(MORPHOLIN-4-YLCARBONYL)PIPERAZIN-1-YL]-5-(3-THIENYL)-1,7-NAPHTHYRIDINE](https://img.chemicalbook.in/StructureFile/ChemBookStructure21/GIF/CB2814380.gif)
![4-[(5-(3-[4-(MORPHOLIN-4-YLCARBONYL)PIPERAZIN-1-YL]PROPYL)PYRIDIN-3-YL)CARBONYL]MORPHOLINE](https://img.chemicalbook.in/StructureFile/ChemBookStructure21/GIF/CB2801326.gif)
You may like
-
 N,N-Diethyl-4-methylpiperazine-1-carboxamide 95% CAS 90-89-1View Details
N,N-Diethyl-4-methylpiperazine-1-carboxamide 95% CAS 90-89-1View Details
90-89-1 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1