diethyl benzylphosphonate
Synonym(s):(Diethoxyphosphonomethyl)benzene;Benzylphosphonic acid diethyl ester;NSC 62294
- CAS NO.:1080-32-6
- Empirical Formula: C11H17O3P
- Molecular Weight: 228.22
- MDL number: MFCD00009078
- EINECS: 214-097-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
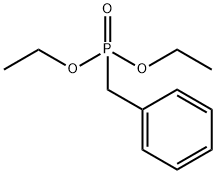
What is diethyl benzylphosphonate?
Chemical properties
CLEAR COLOURLESS TO SLIGHTLY YELLOW LIQUID
The Uses of diethyl benzylphosphonate
Reactant for synthesis of:
3,5-dihydroxy-4-isopropylstilbene for treatment of skin disorders
Natural cytotoxic marine products of polyketide origin via intramolecular Diels-Alder reactions
Stilbenes via on-column oxidation of vicinal diols and Horner-Emmons reactions
Inhibitors of teh Wnt pathway for colon cancer repression using Wadsworth-Emmons reactions
Antimalarial drug analogs against P. falciparum
Reactant for:
Cyclization of aryl ethers, amines, and amides
Investigating the effects of functional groups on the performance of clue organic LEDs
The Uses of diethyl benzylphosphonate
Diethyl benzylphosphonate is used as a reactant for synthesis of 3,5-dihydroxy-4-isopropylstilbene for treatment of skin disorders and natural cytotoxic marine products of polyketide origin via intramolecular Diels-Alder reactions. It is also used as reactant for cyclization of aryl ethers, amines and amides & for investigating the effects of functional groups on the performance of clue organic LEDs.
Preparation
Diethyl benzylphosphonate was synthesized according to the previously published procedure [1]. Benzylphosphonic acid (1 mmol, 172 mg) and triethyl orthoacetate (30 mmol, 5.5 mL) were mixed overnight at 90 °C. The completion of the reaction was monitored by 31P NMR. After the completion of the reaction, an excess of the orthoester was evaporated under reduced pressure. The crude product was purified via a short silica gel column (hexanes/ethyl acetate). The compound 1 was isolated as a transparent oil with 98% yield (0.98 mmol, 224 mg). 1H NMR (400 MHz, DMSO-d6) δ 7.39–7.14 (m, 5H), 3.94 (dq, JH-P = 7.9, JH-H = 7.0, 4H), 3.21 (d, JH-P = 21.6 Hz, 2H), 1.16 (t, JH-H = 7.0 Hz, 6H); 13C NMR (101 MHz, DMSO-d6) δ 132.3 (d, JC-P = 9.0 Hz), 129.7 (d, JC-P = 6.6 Hz), 128.2 (d, JC-P = 3.0 Hz), 126.4 (d, JC-P = 3.4 Hz), 61.3 (d, JC-P = 6.5 Hz), 32.3 (d, JC-P = 135.1 Hz), 16.1 (d, JC-P = 5.8 Hz); 31P NMR (162 MHz, DMSO-d6) δ 26.5. The NMR data are in accordance with those reported in literature [2].
Synthesis Reference(s)
Tetrahedron Letters, 29, p. 1513, 1988 DOI: 10.1016/S0040-4039(00)80339-1
Synthesis, p. 222, 1981 DOI: 10.1055/s-1981-29393
References
[1] DAMIAN TRZEPIZUR. Selective Esterification of Phosphonic Acids.[J]. Molecules, 2021. DOI:10.3390/molecules26185637.
[2] PETER J. THORNTON. Nucleoside Phosphate and Phosphonate Prodrug Clinical Candidates[J]. Journal of Medicinal Chemistry, 2016, 59 23: 10400-10410. DOI:10.1021/acs.jmedchem.6b00523.
[3] GASTON LAVEN. ChemInform Abstract: Preparation of Benzylphosphonates via a Palladium(0)-Catalyzed Cross-Coupling of H-Phosphonate Diesters with Benzyl Halides. Synthetic and Mechanistic Studies.[J]. ChemInform, 2010, 41 40. DOI:10.1002/chin.201040191.
[4] ANNA BRODZKA. The Synthesis and Evaluation of Diethyl Benzylphosphonates as Potential Antimicrobial Agents.[J]. Molecules, 2022. DOI:10.3390/molecules27206865.
Properties of diethyl benzylphosphonate
| Boiling point: | 106-108 °C1 mm Hg(lit.) |
| Density | 1.095 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Sealed in dry,Room Temperature |
| form | Liquid |
| color | Clear colorless to slightly yellow |
| Specific Gravity | 1.10 |
| Water Solubility | Insoluble in water. |
| BRN | 2580931 |
| CAS DataBase Reference | 1080-32-6(CAS DataBase Reference) |
| EPA Substance Registry System | Phosphonic acid, (phenylmethyl)-, diethyl ester (1080-32-6) |
Safety information for diethyl benzylphosphonate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for diethyl benzylphosphonate
diethyl benzylphosphonate manufacturer
Radison Labs Private Limited
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran
![DIETHYL [3-(TRIFLUOROMETHYL)BENZOYL]PHOSPHONATE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB5210349.gif)
![DIETHYL [4-(TRIFLUOROMETHYL)BENZOYL]PHOSPHONATE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB8250100.gif)

You may like
-
 1080-32-6 Diethyl benzylphosphonate 98%View Details
1080-32-6 Diethyl benzylphosphonate 98%View Details
1080-32-6 -
 1080-32-6 99%View Details
1080-32-6 99%View Details
1080-32-6 -
 Diethyl benzylphosphonate 97% CAS 1080-32-6View Details
Diethyl benzylphosphonate 97% CAS 1080-32-6View Details
1080-32-6 -
 Diethyl Benzylphosphonate CAS 1080-32-6View Details
Diethyl Benzylphosphonate CAS 1080-32-6View Details
1080-32-6 -
 Diethyl benzylphosphonate CAS 1080-32-6View Details
Diethyl benzylphosphonate CAS 1080-32-6View Details
1080-32-6 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0