DIENOCHLOR
- CAS NO.:2227-17-0
- Empirical Formula: C10Cl10
- Molecular Weight: 474.64
- MDL number: MFCD00019272
- EINECS: 218-763-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-23 17:25:30
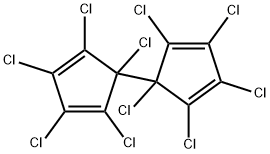
What is DIENOCHLOR?
The Uses of DIENOCHLOR
Dienochlor is used for the control of mites (Tetrunychus spp., Panonychus ulmi and Polyphagotarsonemus latus) on roses, Chrysanthemums and other ornamentals.
The Uses of DIENOCHLOR
Miticide.
The Uses of DIENOCHLOR
Acaricide used for control of mites on ornamentals.
Definition
ChEBI: Dienochlor is an organochlorine compound.
Environmental Fate
Plant. On plants, dienochlor was converted by sunlight to form perchloro ketones (Quistad and Mulholland, 1983).
Chemical/Physical. Dienochlor is unstable when exposed to sunlight. When dienochlor applied as a thin ?lm on glass plates was exposed to sunlight, nonpolar products, a tricyclic chlorocarbon and 3 isomeric perchloro ketones were formed at yields
Dienochlor begins to decompose at 130°C (Worthing and Hance, 1991).
Metabolic pathway
Dienochlor is readily degraded in sunlight to many products, only a few of which have been identified. It is also metabolised in animals but to unknown products. In vitro studies have shown that it interacts with thiols (glutathione, cysteine, efc.) and proteins.
Metabolism
Dienochlor decomposes in simulated sunlight (DT50 1.6 min). Degradation is mainly environmental rather than metabolic, and photochemical breakdown is rapid. It decomposes in soils, DT50 3.1 days and DT50 2–3 days on plants exposed to sunlight.The major degradation products of dienochlor in plants are perchloroketones. It is rapidly degraded in rats.
Degradation
Dienochlor is stable in storage at 54°C for 14 days and at 42°C over
2 years. It undergoes hydrolysis with DT50 values at pH 5,7 and 9 of 184,
93 and 30.5 days, respectively, at 25 °C. It decomposes in simulated sunlight
with a DT50 of 1.6 minutes (PM).
A thin film of dienochlor on glass was readily degraded with a half-life
of <1 hour on exposure to sunlight. Four photoproducts were isolated
from a plethora of products and identified by 13C NMR spectroscopy
(Quistad and Mulholland, 1983). The photocatalysed addition of two
chlorine atoms (from donor dienochlor) afforded the tricyclic photochlorination
product 2 (up to 10% yield). Three isomeric perchloroketones
(3,4 and 5), each resulting from the net addition of one oxygen atom, were
major products, both on glass (up to 14% combined isomers) and on
cucumber and strawberry plants (up to 11% yield). Generally products 2,
3 and 4 were found in similar yields and 5 was the minor of the identified
metabolites. The photochlorination product 2, however, was not detected
on the plants. These products are illustrated in Scheme 1.
Properties of DIENOCHLOR
| Melting point: | 122-123℃ |
| Boiling point: | 547.24°C (rough estimate) |
| Density | 1.8769 (rough estimate) |
| vapor pressure | 2.9 x 10-4 Pa (25 °C) |
| refractive index | 1.6000 (estimate) |
| form | neat |
| Water Solubility | 0.025 mg l-1 |
| Merck | 13,3131 |
| BRN | 2064747 |
| EPA Substance Registry System | Dienochlor (2227-17-0) |
Safety information for DIENOCHLOR
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for DIENOCHLOR
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
