Dibromomethane
Synonym(s):DBM;Dibromomethane;dopamine beta-hydroxylase;dopamine beta-hydroxylase (dopamine beta-monooxygenase);Methylene bromide
- CAS NO.:74-95-3
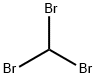
- Empirical Formula: CH2Br2
- Molecular Weight: 173.83
- MDL number: MFCD00000168
- EINECS: 200-824-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13

What is Dibromomethane?
Description
Dibromomethane is a member of the class of bromomethanes that is methane substituted by two bromo groups. It is produced by marine algae. It has a role as a marine metabolite and an algal metabolite. It is a member of bromomethanes and a bromohydrocarbon.
Chemical properties
colourless liquid
Chemical properties
Dibromomethane is a colorless liquid with a sweet, pleasant odor.
The Uses of Dibromomethane
Dibromomethane is used as solvent in organic synthesis. It acts as an intermediate in the manufacture of specialty chemicals, agrochemicals and pharmaceuticals. It is useful as extractant and utilized for the determination of 5-nitroimidazoles (5-NDZ) in environmental waters. It is involved in the convertion of catechols to their methylenedioxy derivatives.
The Uses of Dibromomethane
Organic synthesis, solvent.
Definition
ChEBI: A member of the class of bromomethanes that is methane substituted by two bromo groups. It is produced by marine algae.
General Description
A colorless liquid with a pleasant odor. Insoluble in water and denser than water. May be toxic by ingestion. Used as a solvent and as a motor fuel.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Halogenated aliphatic compounds, such as Dibromomethane, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Low molecular weight haloalkanes are highly flammable and can react with some metals to form dangerous products. Materials in this group are incompatible with strong oxidizing and reducing agents. Also, they are incompatible with many amines, nitrides, azo/diazo compounds, alkali metals (potassium), and epoxides.
Health Hazard
INHALATION: Anesthetic effects, nausea and drunkenness. CONTACT WITH SKIN AND EYES: Skin irritation of eyes and nose.
Fire Hazard
Special Hazards of Combustion Products: Dissociation products generated in a fire may be irritating or toxic.
Toxicology
A poison. Moderately toxic by subcutaneous route. Mdly toxic by inhalation. Mutation data reported. mxtures with potassium explode on light impact. When heated to decomposition it emits toxic fumes of Br-. See also BROMIDES
Hazard
In laboratory studies, animals experienced CNS depression at 2400-2800 ppm and liver and kidney damage after repeated exposures to 1000 ppm. Dichloromethane rarely causes hepatotoxicity unless exposure is extremely heavy or agent ingested. If left on clothes, it may cause reddening of skin; may have effects on the nervous system and blood, causing impaired functions, carboxyhemoglobinemia, and lowering of consciousness; chronic exposure may cause liver and kidney effects; an irritant; harmful by inhalation; a simple asphyxiant; may cause blood disorders, cardiac irregularities, and CNS depression;
Synthesis
Dibromomethane is synthesized commercially from dichloromethane via bromochloromethane:
6 CH2Cl2 + 3 Br2 + 2 Al → 6 CH2BrCl + 2 AlCl3
CH2Cl2 + HBr → CH2BrCl + HCl
The latter route requires aluminium trichloride as a catalyst. The bromochloromethane product from either reaction can further react in a similar manner:
6 CH2BrCl + 3 Br2 + 2 Al → 6 CH2Br2 + 2 AlCl3
CH2BrCl + HBr → CH2Br2 + HCl
In the laboratory, it is synthesized from bromoform:
CHBr3 + Na3AsO3 + NaOH → CH2Br2 + Na3AsO4 + NaBr
using sodium arsenite and sodium hydroxide.
Another way is to synthesize it from diiodomethane and bromine.
Potential Exposure
Methylene bromide is used as a solvent and as a chemical intermediate.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit
Storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed container in a well-ventilatedarea away from potential high heat sources. Where possible,automatically pump liquid from drums or other storage containers to process containers.
Shipping
UN2664 Dibromomethane, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Mixture with potassium forms a shocksensitive explosive. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, sodium amide, strong acids, strong bases, alkaline earth metals, aluminum, magnesium. The substance decomposes on contact with hot surfaces producing hydrogen bromide
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal
Properties of Dibromomethane
| Melting point: | -52 °C |
| Boiling point: | 96-98 °C(lit.) |
| Density | 2.477 g/mL at 25 °C(lit.) |
| vapor density | 6.05 (vs air) |
| vapor pressure | 34.9 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 96-98°C |
| storage temp. | Store below +30°C. |
| solubility | 11.7g/l |
| form | Liquid |
| color | Clear colorless to slightly brown |
| Water Solubility | 0.1 g/100 mL (20 ºC) |
| Merck | 14,6061 |
| BRN | 969143 |
| Dielectric constant | 6.7(10℃) |
| Stability: | Stable. Incompatible with strong oxidizing agents, aluminium, magnesium. Reacts violently with potassium. |
| CAS DataBase Reference | 74-95-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Methane, dibromo-(74-95-3) |
| EPA Substance Registry System | Dibromomethane (74-95-3) |
Safety information for Dibromomethane
| Signal word | Warning |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P501:Dispose of contents/container to..… |
Computed Descriptors for Dibromomethane
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 74-95-3 Dibromomethane 98%View Details
74-95-3 Dibromomethane 98%View Details
74-95-3 -
 Dibromomethane (stabilized with BHT) CAS 74-95-3View Details
Dibromomethane (stabilized with BHT) CAS 74-95-3View Details
74-95-3 -
 Dibromomethane CAS 74-95-3View Details
Dibromomethane CAS 74-95-3View Details
74-95-3 -
 Dibromomethane 95% CAS 74-95-3View Details
Dibromomethane 95% CAS 74-95-3View Details
74-95-3 -
 Dibromomethane CAS 74-95-3View Details
Dibromomethane CAS 74-95-3View Details
74-95-3 -
 Dibromomethane CAS 74-95-3View Details
Dibromomethane CAS 74-95-3View Details
74-95-3 -
 Dibromomethane CAS 74-95-3View Details
Dibromomethane CAS 74-95-3View Details
74-95-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
