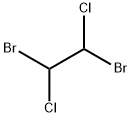
Bromochloromethane
- CAS NO.:74-97-5
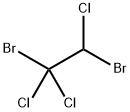
- Empirical Formula: CH2BrCl
- Molecular Weight: 129.38
- MDL number: MFCD00000880
- EINECS: 200-826-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02

What is Bromochloromethane?
Description
Chlorobromomethane is a clear, colorless topale yellow liquid with a chloroform-like odor. Odor threshold=400 ppm. Molecular weight=129.39; Specific gravity (H2O:1)=1.93; Boiling point=68.3℃; Freezing/Melting point=2 88℃; Vapor pressure=115 mmHg at20℃. Hazard Identification (based on NFPA 704 M RatingSystem): Health 2, Flammability 0, Reactivity 0. Insolublein water.
Chemical properties
white to light yellow crystal powder
Chemical properties
Chlorobromomethane is a clear, colorless, to pale-yellow liquid with a chloroform-like odor
Physical properties
Clear, colorless liquid with a sweet, chloroform-like odor
The Uses of Bromochloromethane
Bromochloromethane is used primarily as an extinguishing agent due to its oxygen-depleting properties.
The Uses of Bromochloromethane
Fire fighting agent
Definition
ChEBI: A one-carbon compound substituted by a chloro and a bromo group.
General Description
A clear colorless liquid with a sweet chloroform-like odor. Denser than water (density 1.991 g / cm3) and insoluble in water. Hence sinks in water. Boiling point 68°C. Vapors may cause illness if inhaled. Nonflammable. When exposed to high temperatures may emit toxic fumes. Used as a fire extinguishing agent.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Bromochloromethane is sensitive to light (may discolor). Incompatible with strong bases and strong oxidizing agents. Also incompatible with active metals, calcium, aluminum, magnesium, zinc and their alloys. Attacks some forms of plastics, rubber and coatings. .
Hazard
By inhalation.
Health Hazard
Toxic by ingestion. Vapors may cause dizziness or suffocation. Exposure in an enclosed area may be very harmful. Contact may irritate or burn skin and eyes. Fire may produce irritating and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
Some of these materials may burn, but none ignite readily. Most vapors are heavier than air. Air/vapor mixtures may explode when ignited. Container may explode in heat of fire.
Safety Profile
Mildly toxic by ingestion and inhalation. Mutation data reported. This material has a narcotic action of moderate intensity, although of prolonged duration. Animals exposed for several weeks to 1000 pprn had blood bromide levels as high as 350 mgl100 g. Therefore, until further data are available, it should be considered at least as toxic as carbon tetrachloride and more than minimal exposure to its vapors should be avoided. Dangerous; when heated to decomposition it emits highly toxic fumes of Brand Cl-. See also BROMIDES and CHLORINATED HYDROCARBONS, ALIPHATIC.
Potential Exposure
This compound is used in brominated flame retardants; a fire-extinguishing agent; and in organic synthesis
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, includingresuscitation mask) if breathing has stopped and CPR if heartaction has stopped. Transfer promptly to a medical facility.When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Donot make an unconscious person vomit. Medical observationis recommended for 24-48 h after breathing overexposure,as pulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
Carcinogenicity
The U.S. EPA classification is D (not classifiable as to human carcinogenicity). Bromochloromethane is structurally similar to dichloromethane (methylene chloride), which is classified B2 (probable human carcinogen). The classification is based on the lack of data regarding the carcinogenicity of bromochloromethane in humans or animals; however, there are data indicative of genotoxic effects and structural relationships to halogenated methanes classified as B2 (probable human carcinogens).
Source
No MCLGs or MCLs have been proposed, however, a DWEL of 0.5
mg/L was recommended (U.S. EPA, 2000).
Naturally formed by algal biological processes (Orkin et al., 1997) and is a disinfection
byproduct in public water treatment systems.
Environmental Fate
Biological. When bromochloromethane (5 and 10 mg/L) was statically incubated in the dark at
25 °C with yeast extract and settled domestic wastewater inoculum for 7 d, 100% biodegradation
with rapid adaptation was observed (Tabak et al., 1981).
Photolytic. The following rate constants were reported for the reaction of bromochloromethane
and OH radicals as measured by both flash photolysis resonance fluorescence and discharge flow
electron paramagnetic resonance techniques (x 10-13 cm3/molecule?sec): 0.91 at 4 °C, 1.11–1.13 at
25 °C, 1.32–1.34 at 40 °C, 1.55–1.58 at 57 °C, 1.76–1.90 at 76 °C, 2.10–2.26 at 97 °C (Orkin et
al., 1997).
Chemical/Physical. Although no products were identified, the estimated hydrolysis half-life in
water at 25 °C and pH 7 is 44 yr (Mabey and Mill, 1978). Bromochloromethane reacts with
bisulfide ion (HS-), produced by microbial reduction of sulfate, forming 1,3,5-trithiane and
dithiomethane. Estimated reaction rate constants at 25 and 35 °C were 7.29 x 10-5 and 2.42 x 10-
4/M?sec, respectively (Roberts et al., 1992).
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Chlorobromomethane must be stored to avoid contact with chemically active metals, since violent reactionsoccur. Store in tightly closed containers in a cool, wellventilated area away from heat.
Shipping
UN1887 Bromochloromethane, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Incompatible with strong oxidizers (possible explosion), reducing agents, bases, carbonates, furyl alcohol, chemically active metals, such as calcium; base metals in the presence of moisture, powdered aluminum; zinc, magnesium. Liquid attacks some plastics, rubber, and coatings.
Waste Disposal
Incinerate together with flammable solvent in furnace equipped with afterburner and alkali scrubber.
Properties of Bromochloromethane
| Melting point: | −88 °C(lit.) |
| Boiling point: | 68 °C(lit.) |
| Density | 1.991 g/mL at 25 °C(lit.) |
| vapor density | 4.5 (vs air) |
| vapor pressure | 117 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 11 °C |
| storage temp. | 2-8°C |
| solubility | Soluble in acetone, alcohol, benzene, ether (Weast, 1986), and many other solvents, particularly
chlorinated hydrocarbons. |
| form | neat |
| Specific Gravity | 1.991 |
| Water Solubility | 9 g/L (20 ºC) |
| BRN | 1730801 |
| Henry's Law Constant | (x 10-3 atm?m3/mol):
1.44 at 25 °C (approximate - calculated from water solubility and vapor pressure) |
| Exposure limits | NIOSH REL: TWA 200 ppm (1,050 mg/m3), IDLH 2,000 ppm; OSHA PEL:
TWA 200 ppm; ACGIH TLV: TWA 200 ppm (adopted) |
| Dielectric constant | 7.8(Ambient) |
| Stability: | Stable, but may discolour in light. Incompatible with aluminium, magnesium, zinc, calcium, strong oxidizing agents. |
| CAS DataBase Reference | 74-97-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Methane, bromochloro-(74-97-5) |
| EPA Substance Registry System | Halon 1011 (74-97-5) |
Safety information for Bromochloromethane
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H420:Hazardous to the ozone layer |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Bromochloromethane
New Products
1-Amino-1-cyclohexanecarboxylic acid Cycloleucine 6-Bromo-3-iodo-1-methyl-1H-indazole 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole ELECTROLYTIC IRON POWDER 2-Methyl-2-phenylpropyl acetate 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate 4-Ethylbenzylamine Methyl 5-bromo-2-chloro-3-nitrobenzoate N-(5-Amino-2-methylphenyl)acetamide 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine methyl 6-chloro-2-(chloromethyl)nicotinate 2-methoxy-4-methyl-5-nitro pyridine 2-iodo-5-bromo pyridine 2-amino-4-methyl-5-nitro pyridine 5-Fluoro-2-Oxindole methyl L-alaninate hydrochloride diethyl L-glutamate hydrochloride Ethyl tosylcarbamateRelated products of tetrahydrofuran



You may like
-
 Bromochloromethane CAS 74-97-5View Details
Bromochloromethane CAS 74-97-5View Details
74-97-5 -
 Ethoxymethylenemalononitrile 99% HPLCView Details
Ethoxymethylenemalononitrile 99% HPLCView Details
123-06-8 -
 3-hydroxy-2-nitrobenzoic acid 602-00-6 98%View Details
3-hydroxy-2-nitrobenzoic acid 602-00-6 98%View Details
602-00-6 -
 Diethyl Disulfide 99% HPLCView Details
Diethyl Disulfide 99% HPLCView Details
110-81-6 -
 6285-05-8. 1-(4-chlorophenyl) propan-1-one 98%View Details
6285-05-8. 1-(4-chlorophenyl) propan-1-one 98%View Details
6285-05-8. -
 4-chloro-3,5-dinitropyridine 98%View Details
4-chloro-3,5-dinitropyridine 98%View Details -
 401-95-6 99% HPLCView Details
401-95-6 99% HPLCView Details
401-95-6 -
 171663-13-1 tert-Butyl 3-bromobenzylcarbamate 98%View Details
171663-13-1 tert-Butyl 3-bromobenzylcarbamate 98%View Details
171663-13-1