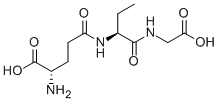
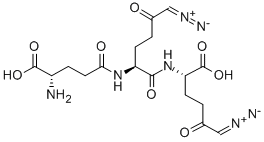
Diazomycin B
- CAS NO.:7644-67-9
- Empirical Formula: C17H23N7O8
- Molecular Weight: 453.41
- MDL number: MFCD01939802
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
What is Diazomycin B?
Originator
Azotomycin,ZYF Pharm
The Uses of Diazomycin B
Antineoplastic.
Manufacturing Process
Azotomycin is anticancer antibiotic produced by Streptomyces ambofaciens.
Total sythesis of it from γ-benzyl-N-tert-butyloxycarbonyl-L-glutamic acid (γ-
OBzl-N-Boc-L-Glu) has been accomplished in nine steps. The mixed carbonic
anhydride method was chosen for peptide bond formation. Commerically
available γ-OBzl-N-Boc-L-Glu was esterified with ethereal diazomethane,
deprotected with trifluoroacetic acid-methylene chloride (1:1), and converted
to hydrochloride γ-benzyl-L-glutamic acid α-methyl ester (γ-OBzl-L-Glu-α-OMe
HCl) by treatment with dry hydrogen chloride in ethyl ether, MP: 129°-135°C
(dec.), [α]D
25= + 13.3° (CHCl3).
Reaction of γ-OBzl-N-Boc-L-Glu with isobutyl chloroformate and Nmethylmorpholine
followed by addition of γ-OBzl-L-Glu-α-OMe HCl afforded
dipeptide γ-OBzl-N-Boc-L-Glu-γ-OBzl-L-Glu-α-OMe, yield 93%, MP: 58.5°-
60°C, [α]D
25= + 7.10° (CHCl3). After cleaving (trifluoroacetic acid-methylene
chloride) the Boc group of above dipeptide, N-Tfa-L-Glu-OMe (Tfatrifluoroacetyl)
was condensed with the product to afford tripeptide N-(γ-NTfa-
L-Glu-α-OMe)-v-OBzl-N-Boc-L-Glu-γ-OBzl-L-Glu-α-OMe as colorless
crystals, MP: 120°-122°C, [α]D
25= + 7.7° (CHCl3). Following hydrogenolysis
of benzyl esters using palladium-on-carbon (10%), diacid N-(γ-N-Tfa-L-Glu-α-
OMe)-L-Glu-L-GluOMe was obtained in yield 94% as colorless crystals, MP:
181°-184°C, [α]D
25= - 37.6° (CH3OH).
Selectively protected above diacid was converted to (γ-N-Tfa-L-Glu-α-OMe)-LGlu-
L-Glu-tetra-OMe next way. To a solution of N-(γ-N-Tfa-L-Glu-α-OMe)-L-Glu-
L-Glu-α-OMe (0.18 g) in dry acetone (20 ml cooled to 0°C was added with
magnetic stirring ethereal diazomethane (0.8 mole) until yellow color
persisted. After evaporation of solvent, the colorless solid was recrystallized
from methylene chloride-hexanes to afford 0.17 g (92%) of colorless crystals
of (γ-N-Tfa-L-Glu-α-OMe)-L-Glu-L-Glu-tetra-OMe, MP: 150°-152°C, [α]D
25= -
8.5° (CHCl3).
A 1.00 g (1.9 mmoles) of (γ-N-Tfa-L-Glu-α-OMe)-L-Glu-L-Glu-tetra-OMe was
dissolved under argon in dry dimethoxyethane (30 ml) with warming and
magnetic stirring, and triethylamine (0.55 ml, 3.97 mmoles) was added. The
reaction mixture was cooled to -30°C (dry ice isopropyl alcohol), and oxalyl
chloride (0.35 ml, 3.97 mmoles) was added followed by dimethylformamide (2drops). The reaction mixture was warmed to 0°C by addition of hot isopropyl
alcohol to the dry ice bath, stirred for 40 min, and then cooled to -78°C.
The cold acid chloride solution was slowly (30 min) added through the
sintered-glass filter into ethereal solution of diazomethane (0.5 moles, 30 ml)
cooled to -23°C (dry ice-carbon tetrachloride). After the mixture was stirred
30 min at -23°C and 30 min at 0°C, solvent was evaporated by steam argon,
and the residue was chromatographed in 19:1 ethyl acetate-methanol on
column of silica gel (70 g). The fraction (24:1 ethyl acetate-methanol) with
TLC Rf 0.16 were collected and solvent evaporated to 0.30 g (38 %) of light
yellow crystals of N-Tfa(-)-azotomycin-di-OMe, which upon recrystallization
from chloroform-ethyl ether, melted at 134°-136°C, [α]D
25= - 22.9°.
To a mixture of N-Tfa(-)-azotomycin-di-OMe (106 mg, 0.18 mmoles) and
methanol (0.12 ml) was added 1.0 N sodium hydroxide (0.83 ml, 8.3 mmoles)
at room temperature and stirred for 30 min. Then the solution was acidified to
pH 6.9 with 0.1 N hydrochloric acid and extracted with chloroform (2x25 ml).
The aqueous phase was dissolved in methanol and passed through a column
of Sephadex LH-20 (250 g), and fractions containing the magor product (TLC
Rf 0.31, methanol, tailing) were collected. Solvent was evaporated and the
residue was freeze-dried to yield 54.3 mg (65%) of (-)-azotomycin as a light
yellow solid, which slowly decomposed at ambient temperature, [α]D
25= -
4.3°, IR, UV, 1H NMR, 13C NMR spectrum confirmed the structures of all
described compounds.
Therapeutic Function
Antineoplastic
Safety Profile
Poison by ingestion,intraperitoneal, subcutaneous, and intravenous routes.Human gastrointestinal tract effects by intravenous route.When heated to decomposition it emits toxic fumes ofNOx.
Properties of Diazomycin B
| Boiling point: | 560.56°C (rough estimate) |
| Density | 1.2516 (rough estimate) |
| refractive index | 1.6800 (estimate) |
Safety information for Diazomycin B
Computed Descriptors for Diazomycin B
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4