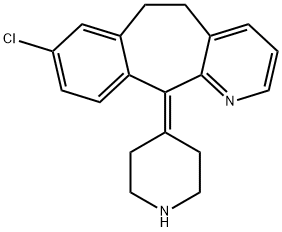
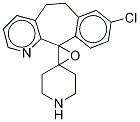
Desloratadine
Synonym(s):8-Chloro-6,11-dihydro-11-(4-piperidinylidene)-5H- benzo[5,6]cyclohepta[1,2,b]pyridine;Clarinex;Desloratadine;NSC 675447
- CAS NO.:100643-71-8
- Empirical Formula: C19H19ClN2
- Molecular Weight: 310.82
- MDL number: MFCD00871949
- EINECS: 638-878-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
What is Desloratadine?
Absorption
Desloratadine administered orally for ten days to healthy volunteers as a 5 mg tablet once daily resulted in a mean Tmax of approximately 3 hours, a mean steady-state Cmax of 4 ng/ml, and a mean steady-state AUC of 56.9 ng*hr/ml. A similar profile was observed using 10 ml of an oral solution containing 5 mg of desloratadine. Food was found not to affect desloratadine absorption.
Toxicity
Information regarding desloratadine overdose is limited, although somnolence has been reported. In case of overdose, symptomatic and supportive treatment, including removing the unabsorbed drug, is recommended; note, however, that desloratadine and its active metabolite 3-hydroxydesloratadine cannot be eliminated by hemodialysis.
In animal studies, lethality was observed at or above doses of 250 mg/kg in rats and of 353 mg/kg in mice (oral LD50), doses that represent 120 and 290 times the human exposure based on the recommended daily oral dose. In monkey, no deaths occurred at doses up to 250 mg/kg, representing an exposure roughly 810 times that of the recommended dose in humans.
Description
Desloratadine is a second-generation tricyclic antihistamine that the FDA approved as a treatment for the relief of seasonal allergic rhinitis in patients 12 years or older. It is an H1-receptor antagonist with anti-inflammatory activity, and its affinity to the H1 receptor is higher than most other H1-receptor antagonists. Desloratadine works by blocking a certain natural substance (histamine) produced during an allergic reaction. More importantly, desloratadine has a long-lasting effect and does not cause drowsiness because it does not readily enter the central nervous system. Desloratadine is used to treat nasal and non-nasal symptoms (watery eyes, runny nose, itching eyes/nose, sneezing, and itching) of seasonal allergic rhinitis. It is also used to relieve chronic itching caused by hives.
Chemical properties
Beige Solid
Originator
Sepracor (US)
The Uses of Desloratadine
Nonsedating-type histamine H1-receptor antagonist. An active metabolite of Loratadine. Also inhibits generation and release of inflammatory mediators from basophils and mast cells.
The Uses of Desloratadine
antiinflammatory
The Uses of Desloratadine
For the relief of symptoms of seasonal allergic rhinitis, perennial (non-seasonal) allergic rhinitis. Desloratidine is also used for the sympomatic treatment of pruritus and urticaria (hives) associated with chronic idiopathic urticaria.
The Uses of Desloratadine
Desloratadine has been used to test its effect on embryoid body development in an in vitro gastrulation model of P19C5 stem cells. It has also been used as an antihistamine mimic in rats and to treat lung cancer (A549) and glioblastoma (U87) cells in various cellular studies. It is used in adults and children to relieve hay fever and allergy symptoms, including sneezing, runny nose, and red, itchy, tearing eyes. It also relieves symptoms of urticaria (hives; red, itchy raised areas of the skin), including itching and rash.
What are the applications of Application
Desloratadine is a potent and selective H1-receptor agonist
Background
Desloratadine is a second generation, tricyclic antihistamine that which has a selective and peripheral H1-antagonist action. It is the active descarboethoxy metabolite of loratidine (a second generation histamine). Desloratidine has a long-lasting effect and does not cause drowsiness because it does not readily enter the central nervous system.
Indications
For the relief of symptoms of seasonal allergic rhinitis, perennial (non-seasonal) allergic rhinitis. Desloratidine is also used for the sympomatic treatment of pruritus and urticaria (hives) associated with chronic idiopathic urticaria.
Definition
ChEBI: Loratadine in which the ethoxycarbonyl group attached to the piperidine ring is replaced by hydrogen. The major metabolite of loratidine, desloratadine is an antihistamine which is used for the symptomatic relief of allergic conditions including rhinitis nd chronic urticaria. It does not readily enter the central nervous system, so does not cause drowsiness.
Indications
Desloratadine (Clarinex) is an active metabolite of loratadine. A 5 mg daily dose has been shown to be effective. There is no evidence that it offers any advantage over loratadine. Fexofenadine (like its parent compound terfenadine) may rarely promote cardiac arrhythmias (24). No significant difference in efficacy has been noted in the nonsedating H1 blockers. Combinations of nonsedating antihistamines in the morning and sedating antihistamines in the evening may be more cost effective than increasing doses of the nonsedating agent.
Manufacturing Process
To a solution of 10.9 g (0.1 mole) of ethylchloroformate in 300 ml of
anhydrous benzene is added dropwise, with stirring at room temperature, a
solution of 16.2 g (0.05 M) of 11-(N-methyl-4-piperidylidene)-8-chloro-6,11-
dihydro-5H-benzo-[5,6]-cyclohepta-[1,2-b]-pyridine in 200 ml of benzene.
The solution is stirred and is heated under reflux overnight (16-20 hours). The
mixture is cooled and is poured into ice water and the organic layer is
separated, washed with water, dried, and then concentrated to dryness. The
residue is triturated with petroleum ether and a white solid of 8-chloro-6,11-
dihydro-11-(1-ethoxycarbonyl-4-piperidylidene)-5H-benzo[5,6
]cyclohepta[1,2-b]pyridine having a melting point of 128-130°C is
recrystallized from isopropyl ether after decolorization with decolorizing
carbon.
To 12 g of sodium hydroxide in 30 ml ethyl alcohol (70%) add 6 g of 8-chloro6,11-dihydro-11-(1-ethoxycarbonyl-4-piperidylidene)-5Hbenzo[5,6]cyclohepta[1,2-b]pyridine and reflux with stirring for 24 hours.
After about the first 6-8 hours an additional 30 ml of 70% ethyl alcohol may
be added. Remove about 50% of the solvent by distillation in vacuo. Add a
small amount of ice water and acidify with glacial acetic acid. Extract with
chloroform (6-8 times), since the product precipitates from the acetic acid
solution as a thick emulsion which cannot be filtered. Concentrate the
chloroform extracts to a small volume and precipitate the product with hexane
to give crude 8-chloro-6,11-dihydro-11-(4-piperidylidene)-5Hbenzo[5,6]cyclohepta[1,2-b]pyridine acetic acid salt, m.p. 197-200°C.
Recrystallize from benzene-hexane to obtain the product, m.p. 199-200°C.
Yield 4.0-4.5 g.
brand name
Clarinex (Schering-Plough).
Therapeutic Function
Antiallergic
General Description
Desloratadine, 8-chloro-6,11-dihydro-11-(4-piperdinylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine(Clarinex) is a white to off-white powder that is slightlysoluble in water, but very soluble in ethanol and propyleneglycol. It is the proposed active metabolite loratadine and hasa very similar receptor binding and safety profile. It is indicated for the symptomatic relief of pruritus andreduction in the number and size of hives in chronic idiopathicurticaria patients 6 months of age and older and for therelief of the nasal and nonnasal symptoms of perennial allergicrhinitis (in patients 6 months of age and older) and seasonalallergic rhinitis (in patients 2 years of age and older).
Desloratadine is extensively metabolized to 3-hydroxydesloratadine,also an active metabolite, which is subsequentlyglucuronidated . The cytochrome enzymesresponsible for the formation of 3-hydroxydesloratadinehave not been reported. Coadministration ofdesloratadine with CYP3A4 inhibitors results in marginalincreases in plasma concentrations of desloratadine and3-hydroxydesloratadine, but no significant changes in safetyor efficacy. The mean elimination half-life of desloratadineis about 6 hours, and the drug and its metabolites areeliminated in the urine and feces.
Biochem/physiol Actions
Desloratadine is a selective and nonsedating histamine H1 receptor antagonist, an active metabolite of loratadine (Claritin), used to relieve hay fever and allergy symptoms with less drowsiness than other antihistamines; does not significantly inhibits cardiac K+ channels at clinically achievable blood levels. Free from antimuscarinic/anticholinergic effects.
Clinical Use
Desloratadine was launched as an improved version of Schering-Plough's Claritin? (Loratadine), for the treatment of nasal and non-nasal symptoms of seasonal allergic rhinitis (SAR). Desloratadine can be prepared by a 7-step synthesis starting with a Ritter reaction on 2-cyano-3-picoline and involving successively alkylation with 3- chlorobenzyl chloride, dehydration, Grignard reaction with the piperidine magnesium chloride, intramolecular cyclization in strong acidic medium, and finally demethylation of the piperidine. Desloratadine (the descarboethoxyloratadine) is a biologically active metabolite of the second-generation antihistamine Ioratadine. Desloratadine is a nonsedating competitive histamine H1 receptor antagonist with increased potency and improved safety as compared to Ioratadine. When compared with other H1 antagonists as an inhibitor of histamine-induced calcium flux in CHO cells, desloratadine was found to be more potent than most H1 antagonists (such as the widely used terfenadine, fexofenadine, cetirizine, Ioratadine and astemizole) in this assay. Desloratadine is also a potent antagonist of muscarinic M1 and M3 receptors (not M2) indicating anticholinergic activity. These effects may be the explanation for the unexpected decongestant effects of desloratadine reported in clinical trials. Desloratadine appears to be devoid of significant effects on potassium channels and does not appear to suffer from adverse interaction with cytochrome P450 inhibitors. Clinical studies have shown that it does not induce sedation or cardiac arrhythmias and does not potentiate the effects of alcohol. Apparent total body clearance is in the range of 114-201 l/h and the mean elimination half life is 19-34.6 h in human. Desloratadine is available as 5-mg tablets, which is the once-daily recommended dose for adults and children above 12 years. It may be taken without regard to food. The treatment not only improved symptoms of SAR but also improved patient ratings of nasal congestion. The FDA also has issued an "approvable" letter for this agent for the treatment of chronic idiopathic urticaria (ClU).
Metabolism
Desloratadine is metabolized to the active metabolite 3-hydroxydesloratadine, which is subsequently glucuronidated.
References
[1] https://www.drugbank.ca
[2] Raif S. Geha, Eli O. Meltzer (2001) Desloratadine: A new, nonsedating, oral antihistamine, The Journal of Allergy and Clinical Immunology,107, 751-762
Properties of Desloratadine
| Melting point: | 150-151°C |
| Boiling point: | 467.9±45.0 °C(Predicted) |
| Density | 1?+-.0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: >10mg/mL |
| form | powder |
| pka | 10.27±0.20(Predicted) |
| color | White to Light orange to Yellow |
| Merck | 14,2922 |
| CAS DataBase Reference | 100643-71-8(CAS DataBase Reference) |
Safety information for Desloratadine
Computed Descriptors for Desloratadine
| InChIKey | JAUOIFJMECXRGI-UHFFFAOYSA-N |
Desloratadine manufacturer
SRINI PHARMACEUTICALS PVT LTD
DR ACHARYA LABORATORIES PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![8-Chloro-6,11-dihydro-11-(4-piperidinylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine-d 1-Oxide](https://img.chemicalbook.in/CAS/20130318/GIF/CB22621829.gif)

![8-Chloro-11-fluoro-6,11-dihydro-11-(4-piperidinyl)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine](https://img.chemicalbook.in/CAS/GIF/298220-99-2.gif)
You may like
-
 Desloratadine 99%View Details
Desloratadine 99%View Details -
 DESLORATADINE 99%View Details
DESLORATADINE 99%View Details -
 Desloratadine 99%View Details
Desloratadine 99%View Details -
 Desloratadine 100643-71-8 98%View Details
Desloratadine 100643-71-8 98%View Details
100643-71-8 -
 Desloratadine 99%View Details
Desloratadine 99%View Details -
 Desloratadine 98%View Details
Desloratadine 98%View Details -
 Desloratadine 98% CAS 100643-71-8View Details
Desloratadine 98% CAS 100643-71-8View Details
100643-71-8 -
 Desloratadine Powder API Manufacturer IndiaView Details
Desloratadine Powder API Manufacturer IndiaView Details
100643-71-8
