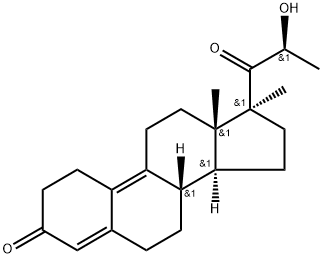
demegestone
- CAS NO.:10116-22-0
- Empirical Formula: C21H28O2
- Molecular Weight: 312.45
- MDL number: MFCD00199859
- EINECS: 2333206
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
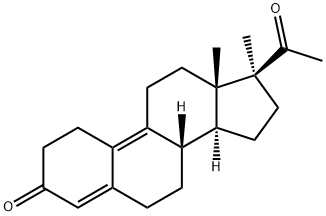
What is demegestone ?
Originator
Lutionex,Roussel,France,1974
Definition
ChEBI: Demegestone is a 20-oxo steroid.
Manufacturing Process
Step A: Preparation of 3-methoxy-17α-methyl-19-nor-?1,3,5(10)-pregnatriene20-one - Under agitation and an inert atmosphere, 1.150 grams of lithium
were introduced into one liter of ammonia cooled to a temperature of -70°C.
For 15 minutes this reaction mixture was agitated, then, while maintaining the
temperature at about -75°C, one liter of ether were added thereto, followed
by 20 grams of 3-methoxy-19-nor-?1,3,5(10),16-pregnatetraene20-one. The
mixture was allowed to stand for 2 hours at a temperature of -75°C under
continued agitation and under continued inert atmosphere. Next, 160 cc of
methyl iodide were added and the reaction mixture was again agitated for 2
hours at -75°C.
Thereafter, the ammonia was evaporated, 1 liter of water was added thereto
and the aqueous phase was separated and extracted with ether. The ethereal
phases now combined were washed with water until the wash waters were
neutral, then dried over sodium sulfate, filtered and distilled to dryness to
obtain 21 grams of product, which was dissolved in 210 cc of ethanol under
reflux. Next, 21 cc of acetic acid and 21 grams of Girard's reactant T were
added thereto. The mixture was agitated for 1? hours under an atmosphere
of nitrogen while maintaining the reflux. Thereafter, the reaction mixture was
cooled to room temperature and then poured into 1,050 cc of water. Next,
155 cc of 2 N sodium hydroxide solution were added and finally the mixture
was extracted with ether.
The combined ethereal phases were washed with water until the wash waters
were neutral, dried over sodium sulfate, filtered and evaporated to dryness to
obtain 16.80 grams of raw product which was purified by redissolving the
product obtained in acetone under reflux and by recrystallization by heating
and cooling.
13.185 grams of 3-methoxy-17α-methyl-19-nor-?1,3,5(10)-pregnatriene-20-one
were thus obtained in the form of a colorless, solid product. The product was
easily soluble in ether, soluble in alcohol, benzene and chloroform and
insoluble in water. This product had a melting point of 109°C and a specific
rotation of [α]D20 = +75°+/-1° (c = 0.5% in chloroform). The starting
compound, 3-methoxy-19-nor-?1,3,5(10),16-pregnatetraene-20-one, was
obtained according to the process described by Burn, J. Chem. SOC.1962,
page 364.
13.185 grams of 3-methoxy-17α-methyl-19-nor-?1,3,5(10)-pregnatriene-20-one
were thus obtained in the form of a colorless, solid product. The product was
easily soluble in ether, soluble in alcohol, benzene and chloroform and
insoluble in water. This product had a melting point of 109°C and a specific
rotation of [α]D20 = +75°+/-1° (c = 0.5% in chloroform). The starting
compound, 3-methoxy-19-nor-?1,3,5(10),16-pregnatetraene-20-one, was
obtained according to the process described by Burn, J. Chem. SOC.1962,
page 364.
The aqueous phase was discarded and the combined ethereal phases were washed with water, dried over sodium sulfate, filtered and distilled to dryness,
to obtain 20.240 grams of 3-methoxy-17α-methyl-19-nor-?2,5(10)-
pregnadiene-20-ol,which product was utilized as such for the next step. The
compound occurred in the form of an amorphous product which was soluble in
alcohol, ether, benzene and acetone and insoluble in water.
washed with water, dried over sodium sulfate, filtered and distilled to dryness,
to obtain 20.240 grams of 3-methoxy-17α-methyl-19-nor-?2,5(10)-
pregnadiene-20-ol,which product was utilized as such for the next step. The
compound occurred in the form of an amorphous product which was soluble in
alcohol, ether, benzene and acetone and insoluble in water.
Step D: Preparation of 17α-methyl-19-nor-?5(10)-pregnene-3,20-dione - 20.5
grams of the compound prepared in Step C were dissolved in 615 cc of
acetone under an atmosphere of nitrogen and under agitation. The solution
obtained was cooled to -20°C. Next 21 cc of a solution of 54 grams of chromic
acid anhydride and 46 cc of dilute sulfuric acid were added thereto. The
solution was allowed to stand for 1 hour under agitation at about -10°C. It
was then poured into 2 liters of a mixture of ice and water and extracted with
benzene. The combined organic phases were washed first with water, then
with a saturated solution of sodium bicarbonate and again with water. Next
these phases were dried over magnesium sulfate and distilled to dryness.
20.40 grams of crude product were thus obtained, which was purified by
subjecting it to chromatography through magnesium silicate and elution with
benzene containing 2.5% of acetone, and recrystallization from isopropyl
ether to obtain 8.50 grams of 17α-methyl-19-nor-?5(10)-pregnene-3,20-dione
in the form of a colorless crystallized product. This product was soluble in
alcohol, ether, acetone, benzene and chloroform and insoluble in water. This
product had a melting point of 138°C, and a specific rotation of [α]D20 =
+168.5°+/-3.5° (c= 0.50% in chloroform).
Step E: Preparation of 17α-methyl-19-nor-?4,9-pregnadiene-3,20-dione -
Under agitation and an atmosphere of nitrogen, 8.50 grams of the compound
prepared in Step D were dissolved in 85 cc of pyridine and cooled to 0C. Next,
16.3 cc of a 29% bromine solution in methanol were added thereto. The
agitation was continued for 30 minutes at the temperature of 0°C. Thereafter
the temperature was raised to room temperature and the solution was allowed
to stand for 16 hours under agitation. The solution was then poured into 850
cc of a mixture of ice and water and after 82 cc of hydrochloric acid were
added, the mixture was extracted with methylene chloride. The combined
organic phases were washed with water until the wash waters were neutral,
then dried over magnesium sulfate and finally distilled to dryness to obtain
8.480 grams of a crude product which was purified by recrystallization from
isopropyl ether. In this manner, 5.810 grams of 17α-methyl-19-nor-?4,9-
pregnadiene-3,20-dione having a melting point of 106°C and a specific rotation [α]D20 = -270+/-4.5° (c = 0.5% in ethanol) were obtained.
Therapeutic Function
Progestin
Properties of demegestone
| Melting point: | 106° |
| alpha | D -275° (c = 0.5 in ethanol) |
Safety information for demegestone
Computed Descriptors for demegestone
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![PROMEGESTONE, [17 ALPHA-METHYL-3H]-](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB1257418.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8