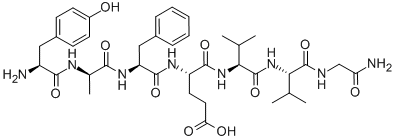
DELTORPHIN II
Synonym(s):Tyr-D -Ala-Phe-Glu-Val-Val-Gly amide
- CAS NO.:122752-16-3
- Empirical Formula: C38H54N8O10
- Molecular Weight: 782.88
- MDL number: MFCD00080072
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-10 18:06:03
What is DELTORPHIN II?
The Uses of DELTORPHIN II
[D-Ala2]-Deltorphin II (cas# 122752-16-3) is a useful research chemical.Deltorphin II is one of the naturally occurring peptides with high affinity and selectivity for δ-??opioid binding sites. Deltorphin II showed even higher affinity for δ-??opioid receptors than previously characterized deltorphins. Also, Deltorphin II were identified as new transport substrates of OATP-??A
Definition
ChEBI: Deltorphin B is a peptide.
Biological Activity
[d-ala2]-deltorphin ii (dadelt ii) is a natural agonist of δ opioid receptor with ki or ic50 value of 0.41 nm [1, 2].opioid receptors are most abundant in the central nervous system, but are also localized in many peripheral tissues in mammalian organisms. there are three types of opioid receptors that are well-defined; the μ, δ and κ receptors [2].[d-pen2, d-pen5]enkephalin (dpdpe) is a second highly selective agonist of δ [3]. treatment with dpdpe at 1 m for as short as 3 min significantly diminished the inhibition of the opioid receptor in ng108-15 cells to cyclic amp production. that means neuronal delta opioid receptor underwent acute desensitization. this desensitization was temporally parallel to the phosphorylation of delta opioid receptor 3 min. after agonist stimulation [4].i.c.v. administration of dadelt ii from 0.38-12.78 nm resulted in a dose- and time-related antinociception. 10 min after the administration of dadelt ii, the maximal antinociceptive response was seen. until 60 min after the administration of dadelt ii, effects were detected. these antinociceptive effects of i.c.v. dadelt ii were antagonized by the selective δ antagonist, ici 174,864 [3]. after supraspinal and spinal administration in rats intracerebroventricularly, dadelt ii at 0.2, 1, 10 μg/rat in a dose-related fashion inhibited diarrhea and colonic bead expulsion but did not show any effect on the rate of small intestine transit. spinal administration of dadelt ii at the same dose produced similar results. naltrindole is a selective antagonist of the δ opioid receptor. subcutaneous pretreatment with naltrindole at 1 and 10 mg/kg antagonized effects of dadelt ii partially and completely, respectively [1].
Biochem/physiol Actions
Selective δ2 opioid receptor agonist
storage
Store at -20°C
References
[1]. broccardo m, improta g. antidiarrheal and colonic antipropulsive effects of spinal and supraspinal administration of the natural δ opioid receptor agonist,[d-ala2] deltorphin ii, in the rat. european journal of pharmacology, 1992, 218(1): 69-73.
[2]. janecka a, fichna j, janecki t. opioid receptors and their ligands. current topics in medicinal chemistry, 2004, 4(1): 1-17.
[3]. jiang q, mosberg hi, porreca f. antinociceptive effects of [d-ala2] deltorphin ii, a highly selective δ agonist in vivo. life sciences, 1990, 47(11): pl43-pl47.
[4]. cai y, zhang y, wu y, et al. δ opioid receptor in neuronal cells undergoes acute and homologous desensitization. biochemical and biophysical research communications, 1996, 219(2): 342-347.
Properties of DELTORPHIN II
| Boiling point: | 1256.2±65.0 °C(Predicted) |
| Density | 1.272±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Soluble to 1 mg/ml in 10% acetonitrile |
| form | solid |
| pka | 4.43±0.10(Predicted) |
| color | White |
| Water Solubility | Soluble to 1 mg/ml in water |
Safety information for DELTORPHIN II
Computed Descriptors for DELTORPHIN II
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


![DELTORPHIN II, (2-D-ALA), [TYROSYL-3,5-3H]-](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB6197481.gif)

![Deltorphin II [D-Ala2]](https://img.chemicalbook.in/)
You may like
-
![[D-Ala2]-Deltorphin II CAS 122752-16-3](https://img.chemicalbook.in//Content/image/CP5.jpg) [D-Ala2]-Deltorphin II CAS 122752-16-3View Details
[D-Ala2]-Deltorphin II CAS 122752-16-3View Details
122752-16-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8