daprodustat,GSK1278863
- CAS NO.:960539-70-2
- Empirical Formula: C19H27N3O6
- Molecular Weight: 393.43
- MDL number: MFCD29924726
- EINECS: 691-659-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-05 12:01:54
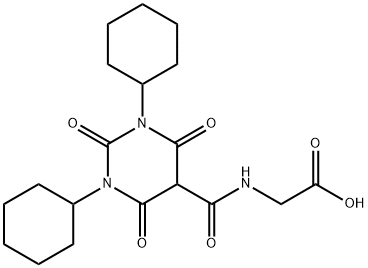
What is daprodustat,GSK1278863?
Absorption
Daprodustat exposure generally increases in a dose-proportional manner over the range of therapeutic doses. Steady-state concentrations are achieved within 24 hours of dosing. Following oral administration, daprodustat is readily absorbed with a median time to peak concentration (Tmax) in healthy subjects ranging from one to four hours. The absolute bioavailability of daprodustat is 65%. Administration of daprodustat with a high-fat or high-calorie meal did not significantly alter daprodustat exposure compared to administration in the fasted state.
Toxicity
Headache and gastrointestinal adverse reactions (e.g., nausea) may be seen with acute overdose with daprodustat. There is no specific antidote. Hemodialysis will not substantially remove daprodustat because it is highly protein bound.
The Uses of daprodustat,GSK1278863
Daprodustat is a novel HIF (Hypoxia inducible factor)-prolyl hydroxylase inhibitor.
Indications
Daprodustat is a hypoxia-inducible factor prolyl hydroxylase (HIF PH) inhibitor indicated for the treatment of anemia due to chronic kidney disease in adults who have been receiving dialysis for at least four months.
The US prescribing information for daprodustat indicates that the drug was not shown to improve quality of life, fatigue, or patient well-being. It is not advised to be used as a substitute for transfusion in patients requiring immediate correction
of anemia. It is also not indicated in patients not on dialysis.
Background
Daprodustat is a small-molecule hypoxia-inducible factor (HIF) prolyl hydroxylase (PHD) inhibitor that was developed by GSK. Patients with CKD cannot induce erythropoietin (EPO) production in response to hypoxia or anemia. As a potent inhibitor of PHD1, PHD2 and PHD3 (≥?1000-fold selectivity), daprodustat stabilizes cellular HIF1α and HIF2α and the induces erythropoiesis. A phase 3 clinical trial (NCT02879305) found that in patients with CKD undergoing dialysis, daprodustat was non-inferior to erythropoiesis-stimulating agents regarding the change in the hemoglobin level from baseline and cardiovascular outcomes.
In June 2020, daprodustat was first approved in Japan for the treatment of renal anemia. On October 2022, the FDA Cardiovascular and Renal Drugs Advisory Committee (CRDAC) supported that the benefit of treatment with daprodustat outweighs the risks for adult dialysis patients with anemia of CKD but not for non-dialysis patients with anemia of CKD. On February 1, 2023, daprodustat was fully approved by the FDA as the first oral treatment for anemia caused by chronic kidney disease in patients on dialysis. The drug is currently under EMA review.
Biological Activity
daprodustat (gsk1278863) is a hif-prolyl hydroxylase inhibitor.hypoxia stimulates erythropoietin (epo) release through accumulation of hypoxia-inducible factor 1α (hif-1α), resulting in an increase of erythrocyte production by the bone marrow. hif-prolyl hydroxylase inhibitors have been used to selectively activate the phd-signaling pathway, leading to the stabilization of hifα subunits, which can affect the transcription of hif-responsive genes.
Pharmacokinetics
Daprodustat is Daprodustat increases endogenous erythropoietin in a dose-dependent manner within six to eight hours after administration. With repeated doses, peak increases in reticulocyte counts occurred in seven to 15 days, with subsequent increases in red blood cell production. New hemoglobin steady-state levels are reached several weeks (approximately four weeks in ESA-users and approximately 16-20 weeks in ESA-non-users) after initial administration.
Daprodustat also increased serum transferrin and total iron binding capacity (TIBC) and decreased serum ferritin, transferrin saturation, and hepcidin when administered for 52 weeks in adults on dialysis with anemia due to CKD.
in vitro
in-vitro biotransformation data indicated that gsk1278863 was primarily metabolized by cyp2c8 [1].
Metabolism
In vitro, daprodustat is primarily metabolized by CYP2C8 (95% contribution), with a minor contribution by CYP3A4 (5%). Following oral or intravenous administration of radiolabeled daprodustat to healthy adults, approximately 40% of the total circulating radioactivity in plasma was daprodustat, and the remaining 60% was metabolites.
The parent drug is the principal circulating component in plasma. Of the six metabolites of daprodustat that were characterized, the major metabolites were M2 (GSK2391220), M3 (GSK2506104), and M13 (GSK2531401), with each metabolite accounting for more than 10% of circulating radioactivity in plasma. In humans, each metabolite circulates primarily as a single stereoisomeric form. In vitro and non-clinical studies suggest that these identified metabolites have a comparable pharmacological activity to the parent drug; however, the extent of the pharmacological contribution of each metabolite is unknown.
References
[1] hara k,takahashi n,wakamatsu a,caltabiano s. pharmacokinetics, pharmacodynamics and safety of single, oral doses of gsk1278863, a novel hif-prolyl hydroxylase inhibitor, in healthy japanese and caucasian subjects. drug metab pharmacokinet.2015 dec;30(6):410-8.
[2] brigandi ra,johnson b,oei c,et al. a novel hypoxia-inducible factor-prolyl hydroxylase inhibitor (gsk1278863) for anemia in ckd: a 28-day, phase 2a randomized trial. am j kidney dis.2016 jun;67(6):861-71.
Properties of daprodustat,GSK1278863
| Melting point: | 237-241oC |
| Density | 1.359±0.06 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), DMSO (Slightly) |
| form | Solid |
| pka | 3.44±0.10(Predicted) |
| color | White to Off-White |
Safety information for daprodustat,GSK1278863
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P362:Take off contaminated clothing and wash before reuse. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for daprodustat,GSK1278863
Abamectin manufacturer
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 960539-70-2 Daprodustat 98%View Details
960539-70-2 Daprodustat 98%View Details
960539-70-2 -
 960539-70-2 99%View Details
960539-70-2 99%View Details
960539-70-2 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4