Dantrolene
- CAS NO.:7261-97-4
- Empirical Formula: C14H10N4O5
- Molecular Weight: 314.25
- MDL number: MFCD00867709
- EINECS: 230-684-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
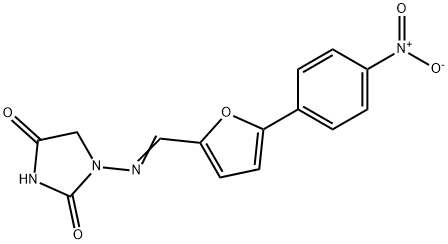
What is Dantrolene?
Absorption
Bioavailability is 70%.
Toxicity
Oral LD50 in rats is 7400 mg/kg. Symptoms which may occur in case of overdose include, but are not limited to, muscular weakness and alterations in the state of consciousness (e.g., lethargy, coma), vomiting, diarrhea, and crystalluria.
Originator
Dantrium,Norwich Eaton ,US,1974
The Uses of Dantrolene
Dantrolene acts as a skeletal muscle relaxant through inhibition of Ca2+ release through ryanodine receptor channels.
The Uses of Dantrolene
Relaxant (skeletal muscle).
Indications
For use, along with appropriate supportive measures, for the management of the fulminant hypermetabolism of skeletal muscle characteristic of malignant hyperthermia crises in patients of all ages. Also used preoperatively, and sometimes postoperatively, to prevent or attenuate the development of clinical and laboratory signs of malignant hyperthermia in individuals judged to be malignant hyperthermia susceptible.
Background
Chemically, dantrolene is a hydantoin derivative, but does not exhibit antiepileptic activity like other hydantoin derivates such as phenytoin.
Definition
ChEBI: The hydrazone resulting from the formal condensation of 5-(4-nitrophenyl)furfural with 1-aminohydantoin.
Manufacturing Process
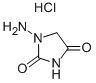
5-(p-Nitrophenyl)-2-furaldehyde (40.0 grams, 0.2 mol) is dissolved in dimethylformamide. An aqueous solution of 1-aminohydantoin hydrochloride (30.0 grams, 0.2 mol) is added. The solution is chilled and diluted with water. The crude material is collected and recrystallized from aqueous dimethylformamide to yield 10.0 grams (16%). MP 279°-280°C. This compound is then converted to the sodium salt.
brand name
Dantrium (Procter & Gamble).
Therapeutic Function
Muscle relaxant
General Description
Crystals (in aqueous DMF).
Air & Water Reactions
Slightly water soluble. Dantrolene may be sensitive to heat and air .
Reactivity Profile
A nitrated amine derivative. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Health Hazard
SYMPTOMS: When ingested, symptoms of exposure may include drowsiness, dizziness, weakness, general malaise, fatigue, diarrhea, constipation, gastrointestinal bleeding, anorexia, abdominal cramps, hepatitis, speech disturbance, seizure, headache, light-headedness, insomnia, tachycardia, phlebitis, mental depression, mental confusion, nervousness, abdominal hair growth, rash, sweating, backache, chills and fever.
Fire Hazard
Flash point data for Dantrolene are not available. Dantrolene is probably combustible.
Pharmacokinetics
Dantrolene is classified as a direct-acting skeletal muscle relaxant. It is currently the only specific and effective treatment for malignant hyperthermia. In isolated nerve-muscle preparation, Dantrium has been shown to produce relaxation by affecting the contractile response of the muscle at a site beyond the myoneural junction. In skeletal muscle, Dantrium dissociates excitation-contraction coupling, probably by interfering with the release of Ca2+ from the sarcoplasmic reticulum. In the anesthetic-induced malignant hyperthermia syndrome, evidence points to an intrinsic abnormality of skeletal muscle tissue. In selected humans, it has been postulated that “triggering agents” (e.g.,general anesthetics and depolarizing neuromuscular blocking agents) produce a change within the cell which results in an elevated myoplasmic calcium. This elevated myoplasmic calcium activates acute cellular catabolic processes that cascade to the malignant hyperthermia crisis. It is hypothesized that addition of Dantrium to the “triggered” malignant hyperthermic muscle cell reestablishes a normal level of ionized calcium in the myoplasm.
Metabolism
Hepatic, most likely by hepatic microsomal enzymes. Its major metabolites in body fluids are 5-hydroxydantrolene and an acetylamino metabolite of dantrolene. Another metabolite with an unknown structure appears related to the latter. Dantrium may also undergo hydrolysis and subsequent oxidation forming nitrophenylfuroic acid.
Properties of Dantrolene
| Melting point: | 279-280° |
| Boiling point: | 453.94°C (rough estimate) |
| Density | 1.3452 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | Store at -20°C |
| solubility | DMSO (Slightly), Methanol (Very Slightly, Sonicated) |
| pka | 7.68±0.10(Predicted) |
| form | Solid |
| color | Yellow to Dark Yellow |
| Stability: | Hygroscopic |
| EPA Substance Registry System | Dantrolene (7261-97-4) |
Safety information for Dantrolene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for Dantrolene
Abamectin manufacturer
SETV ASRV LLP
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
![2-[1-(Aminocarbonyl)-2-[[5-(4-nitrophenyl)-2-furanyl]methylene]hydrazinyl]-acetic Acid Ethyl Ester](https://img.chemicalbook.in/CAS/20180703/GIF/55227-60-6.gif)

You may like
-
 Dantrolene Sodium 7261-97-4 95-99%View Details
Dantrolene Sodium 7261-97-4 95-99%View Details
7261-97-4 -
 Dantrolene CAS 7261-97-4View Details
Dantrolene CAS 7261-97-4View Details
7261-97-4 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4