D(+)-Xylose
Synonym(s):D- (+)-Xylose
- CAS NO.:58-86-6
- Empirical Formula: C5H10O5
- Molecular Weight: 150.13
- MDL number: MFCD00151475
- EINECS: 200-400-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
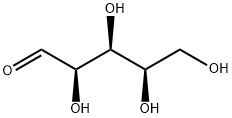
What is D(+)-Xylose?
Absorption
When 12 normal healthy subjects were given an intravenous D-xylose dosing of 10 grams and then an oral dose of 25 grams a week later, the observed absorption percentage was about 69.4% (p < 0.002) and the observed absorption rate was approximately 1.03/hr (p< 0.05) .
The maximum concentration observed in the subjects was 0.53 mg/L with 71 minutes being the time to reach the maximum concentration . The absolute bioavailability recorded was 69% .
Toxicity
Although many national health agencies like the FDA and Health Canada have concluded that the addition or use of food additive sweeteners like xylose is safe and effective for their intended purposes of use in food, it is also known that eating too much of these substances can also ultimately cause gastrointestinal discomfort and laxative effects . These effects are largely the result of excessive amounts of ingested sugar alcohols being poorly taken up from the gastrointestinal tract . Regardless, the extent to which these kinds of effects occur depends on variances between individuals and it is also possible for individuals to develop a tolerance via frequent consumption of such sweetener containing products . In doing so, such individuals can increase consumption of these agents without experiencing adverse effects .
The acute oral toxicity (LD50) for the mouse animal model has been recorded as 23000 mg/kg .
Chemical properties
white crystals or powder
Chemical properties
D-Xylose is nearly odorless and has a smoky flavor. This compound is an aldopentose monosaccharide.
The Uses of D(+)-Xylose
In tanning, dyeing, and as a diabetic food.
The Uses of D(+)-Xylose
sweetener, pharmaceutical intermediate
The Uses of D(+)-Xylose
D-Xylose is used in diagnostic malabsorption tests as well as in the production of Furfural.
Background
Xylose is a monosaccharide of the aldopentose type consisted of five carbon atoms and an aldehyde functional group. Xylose is a sugar isolated from wood. D-Xylose is a sugar widely used as a diabetic sweetener in food and beverage. Xylose has also been used as a diagnostic agent to observe malabsorption. Reduction of xylose by catalytic hydrogenation produces the common food additive sweetener substitute xylitol Xylitol.
The dextrorotary form of xylose, D-xylose, refers usually to the endogenously occurring form of the sugar in living things. The levorotary form, L-xylose, can refer to the form that is synthesized. Nevertheless, xylose by itself may not necessarily serve many purposes immediately - but its metabolism results in a variety of substrates that can serve important nutritional and biological purposes.
Definition
ChEBI: The pyranose form of D-xylose.
Indications
The predominant everyday nutritional usage of xylose is as a parent sugar alcohol from which another sugar alcohol - xylitol- can be derived from and used as an extremely common food additive or sweetener to be used in place of regular sugars as a lower calorie alternative .
Alternatively, xylose was also involved in a procedure known as a D-xylose absorption test that used to be employed to evaluate how well an individual was capable of absorbing a simple sugar like D-xylose from the intestines . By measuring the amount of D-xylose in urine and blood samples after an individual ingested a certain amount of the simple sugar dissolved in some water, the test sought to determine if nutrients were being properly absorbed in the patient's gastrointestinal tract .
What are the applications of Application
D-(+)-Xylose is an aldopentose monosaccharide
Definition
A SUGAR that has five carbon atoms in its molecules.
brand name
Xylo-Pfan (Savage).
General Description
Xylose is a five-carbon sugar that contributes to lignocellulose in plants. Xylose is predominantly found in hardwoods and agricultural residues.
Biochem/physiol Actions
Estimation of xylose in the urine after oral administration, is useful in diagnosing absorption of carbohydrates and malabsorption of non-pancreatic molecules. Xylose plays a significant role in the biologically conversion of plant biomass to fuels and chemicals.
Pharmacokinetics
Xylose is often used as a parent sugar alcohol from which the commonly used food additive sweetener, xylitol, can be derived via the hydrogenation of xylose. Xylitol possesses many characteristics that make it a healthy and effective alternative to regular sugar. For example, although it looks and tastes exactly like ordinary sugar , having a 100% relative sweetness versus normal sucrose , it also has a low impact on blood sugar and insulin secretion and a minimal caloric value of 2.4 calories/gm . Furthermore, xylitol is non-fermentable and thus cannot be transformed to acids by oral bacteria, allowing it to restore a proper alkaline/acid balance in the mouth . Various studies cite this effect for allowing xylitol products like chewing gum to be effective at reducing dental caries . Altogether, these characteristics make xylose and its xylitol metabolite an effective alternative sweetener for healthy food choices for individuals who may be diabetic or for individuals simply wanting to make healthy dietary choices for their bodies.
Metabolism
The most common and traditional metabolism pathway for xylose is the oxidoreductase pathway (or xylose reductase-xylitol dehydrogenase, XR-XDH pathway) . In this pathway, xylose is first reduced to xylitol using the xylitol dehydrogenase (XDH) enzyme with NADH or NADPH . The resultant xylitol is subsequently oxidized to D-xylulose by the xylitol dehydrogenase (XDH) enzyme while utilizing the cofactor NAD . Finally, the D-xylulose is phosphorylated by an ATP utilizing kinase (xylulose kinase enzyme) to generate D-xylulose-5-phosphate, which serves as an intermediate in the pentose phosphate pathway for nucleotide synthesis .
Purification Methods
-D(+)-Xylose forms needles or prisms (which have a very sweet taste) by slow crystallisation from aqueous 80% EtOH or absolute EtOH, which are then dried at 60o in vacuo over P2O5. Store it in a vacuum desiccator over CaSO4. 1Gram dissolves in 0.8mL H2O. [Bragg & Hough J Chem Soc 4347 1957, Hudson & Yanovsky J Am Chem Soc 39 1029 1917, Monroe J Am Chem Soc 41 1002 1919, Beilstein 1 IV 4223.] In D2O at 31o, 1H NMR showed the following ratios: -pyranose (36.5), -pyranose (63), -furanose + -furanose (~1) [Angyal Adv Carbohydr Chem 42 15 1984, Angyal & Pickles Aust J Chem 25 1711 1972].
Properties of D(+)-Xylose
| Melting point: | 154-158 °C(lit.) |
| alpha | 20 º (c=10, H2O) |
| Boiling point: | 191.65°C (rough estimate) |
| Density | 1.525 |
| refractive index | 20 ° (C=10, H2O) |
| FEMA | 3606 | D-XYLOSE |
| Flash point: | > 100°(212°F) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Fine Crystalline Powder |
| pka | pKa (18°): 12.14 |
| Specific Gravity | 1.535 |
| color | White |
| Odor | Odorless |
| PH | 4.0-6.0 (25℃, 1M in H2O) |
| PH Range | 4.5 - 6.0 |
| optical activity | [α]20/D +20.0±1°, 10 hr, c = 10% in H2O |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.05 λ: 280 nm Amax: 0.05 |
| Merck | 14,10087 |
| BRN | 1562108 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 58-86-6(CAS DataBase Reference) |
| NIST Chemistry Reference | D-Xylose(58-86-6) |
| EPA Substance Registry System | D-Xylose (58-86-6) |
Safety information for D(+)-Xylose
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for D(+)-Xylose
D(+)-Xylose manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 D-Xylose CAS 58-86-6View Details
D-Xylose CAS 58-86-6View Details
58-86-6 -
 D-(+)-Xylose extrapure CAS 58-86-6View Details
D-(+)-Xylose extrapure CAS 58-86-6View Details
58-86-6 -
 D-Xylose CAS 58-86-6View Details
D-Xylose CAS 58-86-6View Details
58-86-6 -
 D-(+)-Xylose CAS 58-86-6View Details
D-(+)-Xylose CAS 58-86-6View Details
58-86-6 -
 D-Xylose 99% CAS 58-86-6View Details
D-Xylose 99% CAS 58-86-6View Details
58-86-6 -
 D-XYLOSE Extra Pure CAS 58-86-6View Details
D-XYLOSE Extra Pure CAS 58-86-6View Details
58-86-6 -
 Xylose CAS 58-86-6View Details
Xylose CAS 58-86-6View Details
58-86-6 -
 D-(+)-Xylose CAS 58-86-6View Details
D-(+)-Xylose CAS 58-86-6View Details
58-86-6
