cyclopropanol
- CAS NO.:16545-68-9
- Empirical Formula: C3H6O
- Molecular Weight: 58.08
- MDL number: MFCD19707103
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-01-08 11:28:09
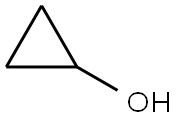
What is cyclopropanol?
Description
Cyclopropanols can be readily synthesized via synthetic transformations such as the Kulinkovich cyclopropanation and the Simmons-Smith reaction [1]. Due to the high strains built intrinsically in the three-membered ring, cyclopropanols and their derivatives are highly reactive and can undergo various ring opening/expansion/fragmentation reactions. A significant amount of these ring opening/expansion/fragmentation reactions are catalyzed/promoted by transition metal complexes. In many of these cases, cyclopropanols and their derivatives are considered as the equivalents of homoenolates or β-alkyl radicals.
The Uses of cyclopropanol
Cyclopropanol is a basic building block.
Definition
ChEBI: Cyclopropanol is a cyclopropane in which a hydrogen atom is replaced by a hydroxy group. It is a member of cyclopropanes and an aliphatic alcohol.
References
[1] Xinpei Cai, Mingji Dai, Weida Liang. “Total syntheses via cyclopropanols.” Tetrahedron 75 2 (2019): Pages 193-208.
Properties of cyclopropanol
| Melting point: | 180℃ |
| Boiling point: | 101-102℃ (760 Torr) |
| Density | 9.9110 g/cm3 |
| refractive index | 1.4129 (589.3 nm 20℃) |
| storage temp. | 2-8°C |
| pka | 15.31±0.20(Predicted) |
| InChI | InChI=1S/C3H6O/c4-3-1-2-3/h3-4H,1-2H2 |
Safety information for cyclopropanol
Computed Descriptors for cyclopropanol
| InChIKey | YOXHCYXIAVIFCZ-UHFFFAOYSA-N |
| SMILES | C1(O)CC1 |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 Cyclopropanol 95% CAS 16545-68-9View Details
Cyclopropanol 95% CAS 16545-68-9View Details
16545-68-9 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4