cyclobutyrol
- CAS NO.:512-16-3
- Empirical Formula: C10H18O3
- Molecular Weight: 186.249
- MDL number: MFCD00129943
- EINECS: 2081385
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
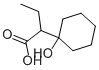
What is cyclobutyrol?
Originator
Hebucol,Logeais,France,1957
Definition
ChEBI: A hydroxy monocarboxylic acid in which the hydroxy group is geminal to a 1-carboxypropyl group on a cyclohexane ring.
Manufacturing Process
Into a balloon flask with two lateral necks furnished with an efficient
mechanical agitator and protected from moisture by a calcium chloride guard,
there are introduced 12 g (0.185 mol) of pure powdered zinc and 20 ml of a
solution of 16.6 g (0.17 mol) of anhydrous cyclohexanone and 31.5 g (0.16
mol) of ethyl α-bromobutyrate in 25 ml of anhydrous benzene. With vigorous
stirring in a manner to put the zinc into suspension, the balloon flask is
gradually heated in an oil bath to 100°C to 105°C. After a few minutes, a
reaction starts, causing violent boiling which is maintained while adding the
balance of the reactants. Boiling is then continued for one hour. After cooling,
the reaction mixture is turned into a beaker containing 30 ml of sulfuric acid
to half (by volume) with ice. After agitation, the mixture is decanted into a
container for separation. The aqueous phase is reextracted with benzene. The
pooled benzene solutions are washed with dilute (10%) cold sulfuric acid, then
with cold sodium carbonate (5%) and then with ice water, and dried over
anhydrous sodium sulfate. The benzene is evaporated and the ester, which is
ethyl α(hydroxy-1-cyclohexyl) butyrate, is distilled off under reduced pressure.
The yield obtained was 17 to 19 g or 49% to 55%.
The ester was saponified with baryta in aqueous methanol as follows:
21.5 g (0.1 mol) of the above ethyl ester is saponified by boiling under reflux
for 4 hours, while agitating, with 30 g (0.095 mol) of barium oxide hydrated
to 8H2O in 250 ml of a mixture of equal volumes of methanol and water. After
concentration to one-half its volume under reduced pressure and filtration, the
aqueous solution is washed with ether and then acidified at 0°C with 10%
hydrochloric acid. The acid liberated in oily form is extracted with ether. The
ether is washed with water, dried and evaporated. The yield is 75-80% (14-15
g of crude acid) which crystallizes spontaneously little by little. It can be
crystallized in a mixture of ether and petroleum ether (1:10) or, with better
yield, in light gasoline or oil (solubility of the pure acid ranges from 0.3% at
0°C to 100% at the boiling point). The yield of crystals is 75-80%. The
α(hydroxy-1-cyclohexyl) butyric acid thus obtained is a colorless crystalline
product with a melting point of 81°C to 82°C.
Therapeutic Function
Choleretic
Properties of cyclobutyrol
| Melting point: | 81-82° |
| Boiling point: | bp24 164°; bp16 167-170° |
| Density | d418.8 1.0010 |
| refractive index | nD18.8 1.4680 (Kon, Naravanan, loc. cit.) |
| pka | 4.69±0.10(Predicted) |
| EPA Substance Registry System | Cyclobutyrol (512-16-3) |
Safety information for cyclobutyrol
Computed Descriptors for cyclobutyrol
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![2-OXO-1-OXA-SPIRO[4.5]DECANE-4-CARBOXYLIC ACID](https://img.chemicalbook.in/CAS/GIF/2819-56-9.gif)

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1