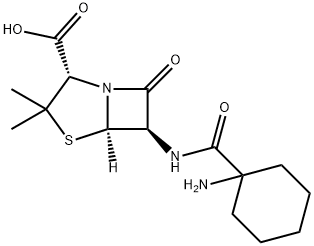
CYCLACILLIN (200 MG)
- CAS NO.:3485-14-1
- Empirical Formula: C15H23N3O4S
- Molecular Weight: 341.43
- MDL number: MFCD00864980
- EINECS: 222-470-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-10 15:15:42
What is CYCLACILLIN (200 MG)?
Absorption
Moderately absorbed.
Toxicity
Symptoms of overdose include severe diarrhea, nausea and vomiting.
Description
Ciclacillin was synthesized by Wyeth Laboratories in 1967 in the course of studies on the improvement of oral absorption of ampicillin. Although its antibacterial activity is one-sixteenth to one-half that of ampicillin, it shows a four to tenfold higher oral absorption and higher urinary excretion. Ciclacillin shows less tendency than ampicillin to cause diarrhea and is used for therapy of pyoderma, wound infection, respiratory and urinary tract infections, as well as ear and nose, and other infections caused by Staphylococcus, Streptococcus, Escherichia coli, Citrobacter, Klebsiella, Proteus, and Haemophilus influenzae.
Originator
UItracillin,Gruenenthal,W. Germany,1972
The Uses of CYCLACILLIN (200 MG)
Antibacterial.
The Uses of CYCLACILLIN (200 MG)
Cyclacillin is an aminosalicylic semisynthetic penicillin; Antibiotic.
Background
A cyclohexylamido analog of penicillanic acid.
Indications
For the treatment of bacterial infections caused by susceptible organisms.
Definition
ChEBI: Cyclacillin is a penicillin. It has a role as an antibacterial drug.
Manufacturing Process
To 21.6 g (0.10 mol) of 6-aminopenicillanic acid (6-APA) and 213 ml of
methylene chloride in a dry 500 ml 3-neck flask fitted with stirrer,
thermometer, nitrogen inlet and reflux condenser with drying tube, 25.3 g
(0.25 mol) of triethylamine and 13.4 g (0.11 mol) of N,N-dimethylaniline were
added. After stirring at reflux for one hour, the mixture was cooled and 21.7 g
(0.20 mol) of trimethylchlorosilane was added dropwise at 12° to 15°C
The mixture was refluxed for 45 minutes, cooled under nitrogen, and 19.8 g
(0.10 mol) of 1-amino-1-cyclohexane-carboxylic acid chloride HCl was added
portionwise at -10°C over 20 minutes. The mixture was stirred for an
additional hour while the temperature rose to 20°C. The reaction mixture was
poured into 200 ml of cold water with stirring and the two-phase mixture
clarified by filtration. Dilute sodium hydroxide solution was added to the
filtrate at 5° to 10°C to pH 5.4.
After stirring overnight at room temperature, the crystalline product was
collected by filtration, washed with water and finally with acetone, and then
dried at 45°C; yield of dihydrate, 29.9 g or 79% of theory based on 6-APA;
iodometric assay, 922 mcg per mg; bioassay, 921 mcg per mg, as described
in US Patent 3,478,018.
brand name
Cyclapen-W (Wyeth).
Therapeutic Function
Antibacterial
Antimicrobial activity
The structure differs from other aminopenicillins in that the benzene ring is completely saturated and the amino substituent is attached directly to it instead of being linked to an adjacent carbon atom. It is less active than ampicillin against staphylococci, streptococci and H. influenzae, but is better absorbed by mouth, peak plasma levels of 10–18 mg/L being reached after a 500 mg oral dose. Its pharmacokinetic properties, side effects and use resemble those of ampicillin. It has limited availability.
Pharmacokinetics
Cyclacillin, a penicillin, is a cyclohexylamido analog of penicillanic acid. Cyclacillin is more resistant to beta-lactamase hydrolysis than ampicillin, is much better absorbed when given by mouth and, as a result, the levels reached in the blood and in the urine are considerably higher than those obtained with the same dose of ampicillin. Cyclacillin has been replaced by newer penicillin treatments.
Metabolism
Not Available
Properties of CYCLACILLIN (200 MG)
| Melting point: | 182-183° (anhydrate) (Hou, Poole); mp 156-158° (dec) (Alburn et al.) |
| Boiling point: | 649.6±55.0 °C(Predicted) |
| alpha | D25 +268° (water) |
| Density | 1.40±0.1 g/cm3(Predicted) |
| solubility | Aqueous Acid (Slightly), DMSO (Slightly), Methanol (Slightly) |
| pka | pK1, pK2 in water: 2.68, 7.50; in 50% dioxane: 4.16, 7.04(at 25℃) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | 32g/L(25 ºC) |
| Stability: | Hygroscopic |
Safety information for CYCLACILLIN (200 MG)
Computed Descriptors for CYCLACILLIN (200 MG)
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4