Crotonic acid
Synonym(s):trans-2-Butenoic acid
- CAS NO.:3724-65-0
- Empirical Formula: C4H6O2
- Molecular Weight: 86.09
- MDL number: MFCD00002701
- EINECS: 223-077-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:39
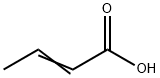
What is Crotonic acid?
Description
Crotonic acid is a white or colorless, crystalline solid with a pungent odor. May be transported as amolten liquid. Molecular weight=86.10; Boilingpoint=187-189℃; Freezing/Melting point=72℃; Flashpoint=88℃ (oc); Autoignition temperature=396℃.Hazard Identification (based on NFPA-704 M RatingSystem): Health 3, Flammability 2, Reactivity 0. Soluble inwater; solubility=9% at 25℃. This chemical is the commercially used trans-isomer. The cis-isomer is lessstable and melts at 15℃.
Chemical properties
Crotonic acid is a white or colorless, crystalline solid with a pungent odor. May be transported as a molten liquid.
The Uses of Crotonic acid
Crotonic acid is used in the synthesis of resins, polymers, plasticizers, and drugs. It is also used as a softening agent for synthetic rubber and in medicinal chemicals.
The Uses of Crotonic acid
Synthesis of resins, polymers, plasticizers, drugs.
Definition
Exists in cis and trans isomeric forms, the latter being the stable isomer used commercially. The cis form melts at 15C and is sometimes called isocrotonic acid.
General Description
A white crystalline solid. Shipped as either a solid or liquid. Melting point 59°F. Soluble in water and less dense than water. Flash point 190°F. Corrosive to metals and tissue.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Crotonic acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Crotonic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.
Hazard
Strong irritant to tissue.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion, skin contact, and subcutaneous routes. A powerful corrosive and irritant. Flammable when exposed to heat or flame; can react with oxidizing materials. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
Used to make plastics, resins, plasticizers, lacquers, and medicines; other chemicals as a chemical intermediate.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek med_x0002_ical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 24-48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
with soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 24-48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
Storage
(1) Color Code—White: Corrosive or ContactHazard; Store separately in a corrosion-resistant location.(2) Color Code—Yellow Stripe (strong reducing agent):Reactivity Hazard; Store separately in an area isolated fromflammables, combustibles, or other yellow-coded materials.(3) Color Code—Blue: Health Hazard/Poison: Store in asecure poison location. Prior to working with crotonic acid,you should be trained on its proper handling and storage.Store in tightly closed containers in a cool, well-ventilatedarea away from oxidizers, strong bases, and reducingagents. Where possible, automatically pump liquid fromdrums or other storage containers to process containers
Shipping
UN2823 Crotonic acid, solid, Hazard class: 8; Labels: 8-Corrosive material.
Incompatibilities
May form explosive mixture with air. A strong reducing agent. The aqueous solution is a weak acid. Violent reaction with oxidizers, combustibles, strong bases; peroxides. Moisture or strong sunlight (UV) may cause explosive polymerization. May accumulate static electrical charges, and may cause ignition of its vapors. Combustible when exposed to heat or flame. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water, and a salt that may be harmful. Incompatible with arsenic compounds 938 Crotonic Acid (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, sulfides (releasing heat, toxic, and possibly flammable gases), thiosulfates, and dithionites (releasing hydrogen sulfate and oxides of sulfur).
Properties of Crotonic acid
| Melting point: | 70-72 °C(lit.) |
| Boiling point: | 180-181 °C(lit.) |
| Density | 1.027 g/mL at 25 °C(lit.) |
| vapor density | 2.97 (vs air) |
| vapor pressure | 0.19 mm Hg ( 20 °C) |
| refractive index | 1.4210 (estimate) |
| FEMA | 3908 | (E)-2-BUTENOIC ACID |
| Flash point: | 190 °F |
| pka | 4.80±0.10(Predicted) |
| Odor | at 0.10 % in propylene glycol. roasted burnt |
| Water Solubility | soluble |
| CAS DataBase Reference | 3724-65-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Crotonic acid(3724-65-0) |
| EPA Substance Registry System | Crotonic acid (3724-65-0) |
Safety information for Crotonic acid
Computed Descriptors for Crotonic acid
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 CROTONIC ACID 99%View Details
CROTONIC ACID 99%View Details -
 Crotonic acid 98%View Details
Crotonic acid 98%View Details -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
