Copper benzoate
- CAS NO.:533-01-7
- Empirical Formula: C14H10CuO4
- Molecular Weight: 305.77
- MDL number: MFCD00035549
- EINECS: 208-552-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
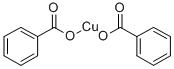
What is Copper benzoate?
Description
Copper benzoate is the chemical compound with the formula Cu(C6H5CO2)2. This coordination complex is derived from the cupric ion and the conjugate base of benzoic acid. Because copper emits blue in a flame, this salt has found some use as a source of blue light in fireworks.
Chemical properties
Blue, crystalline powder; odorless.Slightly soluble in cold water, acids, and alcohol.
Physical properties
Copper (II) benzoates exists in at least two structural forms , depending on the degree of hydration. As for copper (II) acetate , the benzoate adopts a "Chinese lantern" structure, wherein a pair of copper centers are linked by four bridging carboxylate ligands. Typically one site on each copper center is occupied by water, which can be replaced by other ligands . A hydrated form is also known, wherein each Cu (II) center is bound to four water ligands and benzoate .
The Uses of Copper benzoate
Cupric benzoate monohydrate (cas# 533-01-7) is used as a catalyst in the preparation of positive resist compositions comprising sulfonium compounds as acid diffusion inhibitors for high resolution and minimal line edge roughness.
Preparation
In laboratory, copper benzoate can be made by combining aqueous solutions of potassium benzoate with copper sulfate. Copper benzoate precipitates as a pale blue solid :
2 C6H5COOK + CuSO4 →Cu(C6H5COO)2 + K2SO4
The primary use of this of this compound is in production of blue flame in fireworks. Copper benzoate made from sodium benzoate for use in fireworks may result in strong yellow dilution of the flame unless the precipitate is carefully washed to remove sodium ion (which emits brightly yello). Emission from potassium does not complicate the emission spectrum.
Purification Methods
Recrystallise it from hot water. Its solubility in EtOH/*C6H6 (90%) at 25o is 0.1%. [Crawford & Stewart J Chem Soc 228, 289 1953, Beilstein 9 H 84, 9 I 60, 9 III 376, 9 IV 280.]
Properties of Copper benzoate
| EPA Substance Registry System | Benzoic acid, copper(2+) salt (533-01-7) |
Safety information for Copper benzoate
Computed Descriptors for Copper benzoate
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 3-Nitrobenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![bis[4-hydroxysalicylato(2-)-O1,O2]cuprate(2-)](https://img.chemicalbook.in/CAS/GIF/32628-05-0.gif)


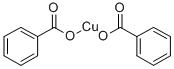
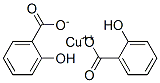
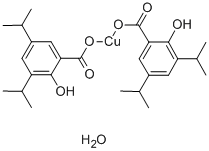
You may like
-
 Copper benzoate 98% (HPLC) CAS 533-01-7View Details
Copper benzoate 98% (HPLC) CAS 533-01-7View Details
533-01-7 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1