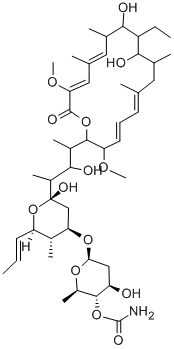
CONCANAMYCIN A
Synonym(s):Concanamycin A;Folimycin;Folimycin, Streptomyces sp. - CAS 80890-47-7 - Calbiochem
- CAS NO.:80890-47-7
- Empirical Formula: C46H75NO14
- Molecular Weight: 866.09
- MDL number: MFCD00210037
- EINECS: 620-709-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-01 14:32:37
What is CONCANAMYCIN A?
Description
Concanamycin A (80890-47-7) is a potent and specific inhibitor of the vacuolar (V-type) H+-ATPase which can induce apoptotic cell death in various cell lines.1,2?Inhibits cell surface expression of virus envelope glycoproteins.3?Dramatically increases the rate of extracellular vesicle release from a variety of cell types.4?Inhibits autophagy by blocking lysosomal acidification.5
The Uses of CONCANAMYCIN A
Concanamycin A is the major analogue of the concanamycin complex produced by Streptomyces sp.. It has been shown to act as a potent and specific vacuolar-ATPase inhibitor. Concanamycin A inhibits the acidification of organelles and blocks cell surface expression of viral envelope glycoproteins without affecting their synthesis. It also interferes with intracellular protein trafficking and inhibits perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. Concanamycins are structurally related to the bafilomycins.
The Uses of CONCANAMYCIN A
Concanamycin A has been used:
- as a lysosomal inhibitor in young and old fibroblasts
- as a vacuolar-type H+-ATPase inhibitor in presynaptic vesicles
- as a lysosomal acidification blocker in HepG2 hepatocytes cells
Definition
ChEBI: A concanamycin in which the lactone ring contains 4 double bonds and is substituted by 4 methyl groups, 2 hydroxy groups, 2 methoxy groups and an ethyl group.
What are the applications of Application
Concanamycin A is a specific inhibitor of vacuolar type H+-ATPase activity (V-ATPase) and is capable of inducing apoptosis
General Description
Chemical structure: macrolide
Biological Activity
Specific inhibitor of V-type (vacuolar) H + -ATPase that displays > 2000-fold selectivity over other H + -ATPases (IC 50 values are 9.2, > 20000, > 20000 and > 20000 nM for yeast V-type, F-type, P-type H + -ATPases and porcine P-type Na + ,K + -ATPase respectively). Blocks cell surface expression of virus envelope glycoproteins without affecting synthesis and exhibits cytotoxicity in several cell lines.
Biochem/physiol Actions
Concanamycin A (ConA) inhibits acidification of organelles and perforin-mediated cytotoxicity. It is a vacuolar-type v-ATPase inhibitor. ConA possesses antiprotozoal and antineoplastic properties. It mediates inhibition of the negative factor (Nef) protein of the human immunodeficiency virus.
storage
Store at -20°C
References
1) Nishihara?et al.?(1995),?Specific inhibitors if vacuolar type H(+)-ATPases induce apoptotic cell death; Biochem. Biophys, Res. Commun.,?212?255 2) Hong?et al. (2006),?Nitric oxide production by the vacuolar-type (H+)-ATPase inhibitors bafilomycin A1 and concanamycin A and its possible role in apoptosis in RAW 264.7 cells; J. Pharmacol. Exp. Ther.,?319?672 3) Muroi?et al.?(1993),?Folimycin (concanamycin A), a specific inhibitor of V-ATPase, blocks intracellular translocation of the glycoprotein of vesicular stomatitis virus before arrival to the Golgi apparatus; Cell Struct. Function,?18?139 4) Cashikar and Hanson (1987),?A cell-based assay for CD63-containing extracellular vesicles; PLoS One,?14?e0220007 5) Gradzka?et al.?(2018),?Inhibitor of apoptosis proteins are required for effective fusion of autophagosomes with lysosomes; Cell Death Dis.,?9?529
Properties of CONCANAMYCIN A
| Melting point: | 179-180℃ (dichloromethane ethanol ) |
| Boiling point: | 966.4±65.0 °C(Predicted) |
| Density | 1.20±0.1 g/cm3(Predicted) |
| storage temp. | −20°C |
| solubility | Soluble in DMSO |
| form | Lyophilized solid |
| pka | 12.46±0.70(Predicted) |
| color | White |
| BRN | 3560277 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. |
Safety information for CONCANAMYCIN A
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for CONCANAMYCIN A
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Concanamycin A CAS 80890-47-7View Details
Concanamycin A CAS 80890-47-7View Details
80890-47-7 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1