Colistin sodium methanesulfonate
Synonym(s):Colimycin sodium methanesulfonate;Colistimethate sodium;Colistin sulphomethate sodium;Methanesulfonic acid derivative of Polymyxin E;Polymyxin E sodium methanesulfonate
- CAS NO.:8068-28-8
- Empirical Formula: Unspecified
- Molecular Weight: 1749.82
- MDL number: MFCD00130824
- EINECS: 232-516-9
- Update Date: 2024-12-18 14:07:02
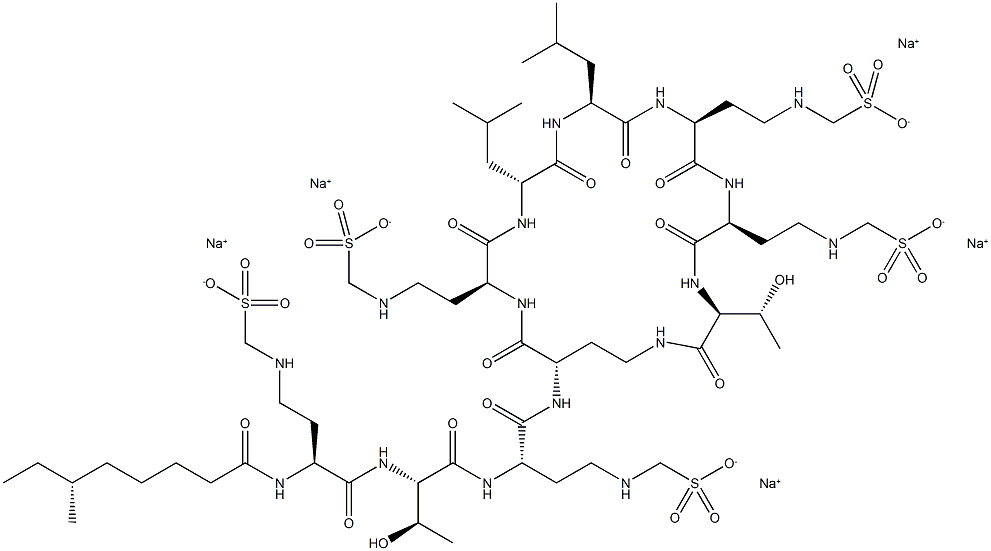
What is Colistin sodium methanesulfonate?
Chemical properties
White or almost white, hygroscopic powder.
The Uses of Colistin sodium methanesulfonate
Colistin sodium methanesulfate is used to permeabilize bacterial cell membranes and to study mannose-resistant haemagglutination. It is used to study effective treatments of P. aeruginosa infections in cystic fibrosis patients and to study the pharmacokinetics and BAL concentration of colistin in critically ill patients.
What are the applications of Application
Colistin sodium methanesulfonate is Colistin sodium methanesulfonate is a cyclopolypeptide antibiotic and derivative of Polymyxin E.
Definition
ChEBI: A mixture where R = H or Me. Colistin in which each of the primary amino groups is converted to the corresponding aminomethanesulfonic acid sodium salt, commonly by the action of formaldehyde followed by sodium bisulfite. A polymyxin antibiotic derivative, it is used in the treatment of severe infections, particularly of multidrug-resistant Gram-negative bacteria such as Pseudomonas aeruginosa and Acinetobacter baumannii.
brand name
Coly-Mycin (Monarch).
General Description
Two forms of colistins, colistin sulfate and colistin methanesulfonate sodium (Colistin sodium methanesulfonate)(CMS-Na), are commercially available. CMS-Na is administered intravenously, and CMS, the free base of CMS-Na consisting of CMS A and CMS B, is hydrolyzed in vivo to form active colistin A and colistin B6 . Colistin first became available for clinical use in 1959 and has been used worldwide for several decades[5].
Biological Activity
colistin methanesulfonate is an inactive prodrug of colistin (also known as polymyxin e), which is an antibiotic with effect against most gram-negative bacteria, but also causes nephro- and neurotoxicity [1]. colistin is produced by certain strains of the bacteria paenibacillus polymyxa, and belongs to the class of polypeptide antibiotics known as polymyxins.in vitro: studying the time-kill kinetics of colistin methanesulfonate against a type culture of pseudomonas aeruginosa found that 8.0 or 32 mg/l colistin methanesulfonate began killing at approximately 90 or 30 min, and the mean auc0-240 values were 186.3±6.0 and 90.4±4.1, respectively [2]. besides, by investigation among mucoid and nonmucoid strains of pseudomonas aeruginosa isolated from patients with cystic fibrosis, colistin methanesulfonate was found to require a concentration of 16 times the mic to achieve complete killing within 24 h [3].
Pharmacokinetics
Colistin is administered intravenously as the non-active prodrug, colistin methanesulfonate sodium (CMS). Colistin methanesulfonate sodium (Colistin sodium methanesulfonate) is eliminated mainly by renal clearance. If CMS is cleared rapidly by the kidney, less CMS is converted to colistin resulted in low colistin that could lead to ineffective antibacterial therapy. In patients with normal renal function (1–2 MIU of CMS), approximately 30–60% of a dose of CMS is converted to colistin. The renal clearance of CMS is much more efficient than the conversion of CMS to colistin. Therefore, to achieve a targeted concentration of >2 mg/L, patients must receive four to five times the amount of CMS. There was wide variability of colistin Cmax values (0.6–8.7 mg/L) in plasma among single-dose studies receiving 2–3 MIU of CMS in critically ill patients with preserved renal function. Almost none of the ICU patients achieved a colistin concentration of more than 2 mg/L in the Plachouras et al. and Mohamed et al. studies[4].
Safety Profile
Human poison by intramuscular route. Experimental poison by intramuscular, intraperitoneal, subcutaneous, and intravenous routes. Mildly toxic by ingestion. An experimental teratogen. Human systemic effects by intramuscular route: convulsions or effect on seizure threshold, change in motor activity, change in lrldney tubules, and urine volume decrease or anuria. Experimental reproductive effects. Used as an antibiotic. When heated to decomposition it emits very toxic fumes of NOx, SOx, and NazO
References
[1] nation r l, li j. colistin in the 21st century.[j]. current opinion in infectious diseases, 2009, 22(6): 535-543.
[2] bergen p j, li j, rayner c r, et al. colistin methanesulfonate is an inactive prodrug of colistin against pseudomonas aeruginosa.[j]. antimicrobial agents and chemotherapy, 2006, 50(6): 1953-1958.
[3] li j, turnidge j d, milne r w, et al. in vitro pharmacodynamic properties of colistin and colistin methanesulfonate against pseudomonas aeruginosaisolates from patients with cystic fibrosis[j]. antimicrobial agents and chemotherapy, 2001, 45(3): 781-785.
[4] Yusuf, Wan Nazirah Wan . "Population Pharmacokinetics of Colistin Methanesulfonate Sodium and Colistin in Critically Ill Patients: A Systematic Review."Pharmaceuticals14(2021).
[5] Mizuyachi, Kaori , et al. "Safety and pharmacokinetic evaluation of intravenous colistin methanesulfonate sodium in Japanese healthy male subjects."Current Medical Research and Opinion27.12(2011):2261-2270.
References
[1] Yusuf, Wan Nazirah Wan . "Population Pharmacokinetics of Colistin Methanesulfonate Sodium and Colistin in Critically Ill Patients: A Systematic Review." Pharmaceuticals 14(2021).
Properties of Colistin sodium methanesulfonate
| Melting point: | >228°C (dec.) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble10mg/mL |
| form | powder |
| color | white to faintly beige |
| PH | 6-8 (10mg/mL in H2O) |
| Water Solubility | Freely soluble in water |
| Merck | 13,2503 |
| CAS DataBase Reference | 8068-28-8 |
| EPA Substance Registry System | Colistimethate sodium (8068-28-8) |
Safety information for Colistin sodium methanesulfonate
Computed Descriptors for Colistin sodium methanesulfonate
Colistin sodium methanesulfonate manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Colistimethate sodium CAS 8068-28-8View Details
Colistimethate sodium CAS 8068-28-8View Details
8068-28-8 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1