COENZYME Q1
Synonym(s):2,3-Dimethoxy-5-methyl-6-(3-methyl-2-butenyl)-1,4-benzoquinone;Ubiquinone-1;Ubiquinone-5
- CAS NO.:727-81-1
- Empirical Formula: C14H18O4
- Molecular Weight: 250.29
- MDL number: MFCD00274412
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
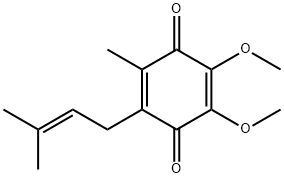
What is COENZYME Q1?
Description
Cooenzyme Q10 (CoQ10) is a component of the electron transport chain and participates in aerobic cellular respiration, generating energy in the form of ATP. CoQ1 is an amphipathic CoQ10 homolog that has a tail consisting of five isoprene units. It has been used as an electron acceptor to study a range of oxidoreductases as isolated enzymes, in subcellular fractions, in intact cells in culture, and in perfused organs. Ubiquinone analogs, including CoQ1, impact mitochondrial permeability transition pore (PTP) formation, as well as PTP-dependent cell death, in an analog- and cell-specific manner.
The Uses of COENZYME Q1
Coenzyme Q1 is an electron acceptor and a component of the electron transport chain. It also play a role in the?mitochondrial permeability transition pore (PTP). Coenzyme Q1 is structurally similar to?coenzyme Q2 (C636450)?and?coenzyme Q10 (C636501).
The Uses of COENZYME Q1
Coenzyme Q1 has been used to measure the mitochondrial respiratory chain complex 1 activity.
What are the applications of Application
Ubiquinone-5 is a CoQ10 analog that counteracts the inhibitory effects on MPTP
Definition
ChEBI: Ubiquinones is any benzoquinone derived from 2,3-dimethoxy-5-methylbenzoquinone; one of a group of naturally occurring homologues. The redox-active quinoid moiety usually carries a polyprenoid side chain at position 6, the number of isoprenoid units in which is species-specific. Ubiquinones are involved in the control of mitochondrial electron transport, and are also potent anti-oxidants. It has a role as an Escherichia coli metabolite and a mouse metabolite. It is a prenylquinone and a member of 1,4-benzoquinones. It is functionally related to a 2,3-dihydroxy-5-methyl-1,4-benzoquinone.
General Description
Coenzyme Q (CoQ) is localized to the hydrophobic domain of the phospholipid bilayer of mitochondria, plasma lipoproteins, and other biological membranes. It is a ubiquinone homolog with a short isoprenoid side chain.
Biochem/physiol Actions
Coenzyme Q1 (CoQ1) is a 1 isoprenyl group (not naturally occurring) member of a family of ubiquinones that share a quinine chemical group but differ in the number of isoprenyl chemical subunits in their tail. The CoQ compounds are lipid soluble components of cell membranes where they perform multiple functions such as electron and proton transport. The most well studied CoQ compound is CoQ10. CoQ1 is frequently used in comparison studies on the effect of isoprenyl chain length on CoQ functions or distribution and to identify quinone reductases.
Properties of COENZYME Q1
| Boiling point: | 389.1±42.0 °C(Predicted) |
| Density | 1.10±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMF: 10 mg/ml; Ethanol: 10 mg/ml |
| form | Orange-red to red liquid. |
| color | Orange to red |
Safety information for COENZYME Q1
Computed Descriptors for COENZYME Q1
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![2,3-DIMETHOXY-5-METHYL-6-[ALL TRANS]FARNESYLFARNESYL-1,4-BENZOQUINONE](https://img.chemicalbook.in/CAS/GIF/1065-31-2.gif)
You may like
-
 Coenzyme Q1 CAS 727-81-1View Details
Coenzyme Q1 CAS 727-81-1View Details
727-81-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8