CMX 001
- CAS NO.:444805-28-1
- Empirical Formula: C27H52N3O7P
- Molecular Weight: 561.69
- MDL number: MFCD09970716
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
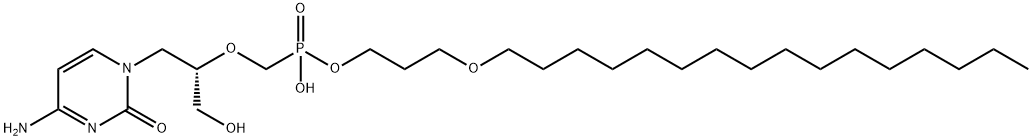
What is CMX 001?
Absorption
The oral bioavailability of brincidofovir is 13.4% in its tablet formulation and 16.8% in its suspension formulation. Following oral administration, the Cmax and AUCtau of brincidofovir were 480 ng/mL and 3400 ng·hr/mL, respectively. The Cmax and AUCtau of the active metabolite, cidofovir diphosphate, were 9.7 pg/106 cells and 1200 pg·hr/106 cells, respectively.
Maximum plasma concentrations (Tmax) of brincidofovir are reached at approximately 3 hours post-administration, while maximal plasma concentrations for cidofovir diphosphate are reached at approximately 47 hours post-administration.
Toxicity
There is no clinical experience with brincidofovir overdose. Patients experiencing overdosage should be monitored closely and provided supportive therapy as clinically indicated.
Description
Remember Ebola? Its predominance in the news has diminished recently, but the need for developing drugs to combat ebolavirus infections is a strong as ever. Brincidofovir is an experimental drug under development by Chimerix (Durham, NC) for combating several viruses, including Ebola. Its activity against Ebola is somewhat unexpected because, unlike other viruses attacked by brincidofovir, Ebola is a single-stranded RNA virus, not a double-stranded DNA virus.
In December 2014,?Doctors without Borders began clinical trials on brincidofovir?in West Africa. Previously, two out of the three Ebola patients treated with the drug in the United States survived, but whether their survival can be attributed to it is not known.
The Uses of CMX 001
Brincidofovir is an antiviral inhibitor, working against BK polymavirus replication in primary human urothelial cells. Cytostatic, cytotoxic agent.
Background
Brincidofovir is an oral antiviral drug used in the treatment of human smallpox infections. It is a lipid conjugate pro-drug of the acyclic nucleotide analogue cidofovir - this lipid conjugate improves drug delivery to the target cells and significantly reduces the nephrotoxicity typically associated with cidofovir therapy. Due to its formulation as a pro-drug brincidofovir also carries a greater bioavailability than cidofovir, allowing for oral administration rather than intravenous. Cidofovir itself has broad antiviral activity against several DNA viruses, resulting in brincidofovir being investigated for the prevention and treatment of cytomegalovirus (CMV), BK Virus (BKV), adenoviruses (AdV), and Epstein-Barr virus (EBV), amongst others.
Brincidofovir, developed by Chimerix under the brand name Tembexa, was approved by the FDA for the treatment of smallpox infection in June 2021. As smallpox has been eradicated, the efficacy of Tembexa was assessed in animals infected with viruses closely related to variola. The approval was granted under the agency’s Animal Rule, which allows for a drug to be approved based on the results of well-controlled animal studies when human trials would be unethical or infeasible.
Indications
Brincidofovir is indicated for the treatment of human smallpox disease in adult and pediatric patients.
Biological Activity
cmx001 is a broad spectrum antiviral agent [1].cmx001 has been reported to be active in vitro against a broad range of viruses from all five families of dsdna viruses infecting human with ec50 values of 0.02μm, 0.0004μm, 17μm , 0.045μm and 0.07μm for adenovirus, herpesvirus, papillomavirus, polyomavirus and orthopoxvirus, respectively. in addition, cmx001 has been revealed to arrest disease progression by inhibiting viral dna replication and thereby reducing viral burden. apart from these, initial studies has been demonstrated the efficacy of cmx001 for pre-exposure and post-exposure prophylaxis in mouse, rabbit, cynomolgus monkeys and human [1].
Pharmacokinetics
The pharmacologically active agent resulting from brincidofovir metabolism, cidofovir diphosphate, has an exceedingly long duration of action that allows for it to be dosed once weekly. The entirety of a brincidofovir smallpox treatment consists of only two doses, on days 1 and 8, which seemingly reduces the risk of adverse reactions. Regimens involving a longer duration of administration (i.e. more than a single dose on days 1 and 8) have been shown to increase mortality compared to placebo and should therefore be avoided. Brincidofovir is considered a potential human carcinogen and has demonstrated the potential to cause infertility - as such, its use should be restricted to situations in which it is absolutely necessary.
Metabolism
Brincidofovir is a pro-drug of cidofovir and as such must undergo some basic metabolic reactions to become pharmacologically active. Upon entering the target cell, the phosphodiester bond of brincidofovir is hydrolyzed to generate cidofovir, which is then phosphorylated to generate the active agent: cidofovir diphosphate. The specific enzyme(s) responsible for this reaction have not been elucidated, but in vitro findings suggest sphingomyelin phosphodiesterase plays a major role in the initial hydrolysis of brincidofovir.
There are two major inactive metabolites of brincidofovir, CMX103 and CMX064, which are generated via carboxylation of the terminal carbon followed by several cycles of CYP-mediated oxidative reactions and fatty acid oxidation. These reactions are mediated, at least in part, by CYP4F2.
References
[1]lanier r1, trost l, tippin t, lampert b, robertson a, foster s, rose m, painter w, o'mahony r, almond m, painter g.development of cmx001 for the treatment of poxvirus infections. viruses. 2010 dec; 2(12):2740-2762.
Properties of CMX 001
| Melting point: | > 177° C (dec.) |
| Boiling point: | 688.6±65.0 °C(Predicted) |
| Density | 1.17±0.1 g/cm3(Predicted) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly, Sonicated), Ethanol (Slightly, Heated, Sonicated), Methanol (Sli |
| form | Solid |
| pka | 2.40±0.10(Predicted) |
| color | White to Off-White |
| Stability: | Hygroscopic |
Safety information for CMX 001
Computed Descriptors for CMX 001
CMX 001 manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 444805-28-1 Brincidofovir 98%View Details
444805-28-1 Brincidofovir 98%View Details
444805-28-1 -
 Brincidofovir CAS 444805-28-1View Details
Brincidofovir CAS 444805-28-1View Details
444805-28-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1