Clortermine
- CAS NO.:10389-73-8
- Empirical Formula: C10H14ClN
- Molecular Weight: 183.68
- MDL number: MFCD00864481
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-22 08:28:48
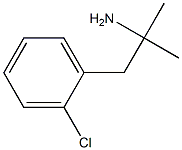
What is Clortermine?
Originator
Voranil,U.S.V.,US,1973
The Uses of Clortermine
Anorexic.
Definition
ChEBI: Clortermine is a member of amphetamines.
Manufacturing Process
To a Grignard reagent (prepared from 50.0 g of o,α-dichloro-toluene and 7.45
g of magnesium in diethyl ether) is added 18.0 g of acetone at such rate that
constant reflux is maintained. The reaction mixture is allowed to stand
overnight at room temperature, and is then poured onto a mixture of 20%
sulfuric acid and ice. The organic layer is separated, washed with water, an
aqueous solution of sodium hydrogen carbonate and again with water, dried
over magnesium sulfate and evaporated to dryness. The residue is distilled
under reduced pressure to yield 42.6 g of 1-(o-chlorophenyl)-2-methyl-2-
propanol, BP 120° to 122°C/12.5 mm.
To 29.0 ml of glacial acetic acid, cooled to 15°C, is added 11.5 g of sodium
cyanide (98%) while stirring, and then dropwise 32.4 ml of concentrated
sulfuric acid, dissolved in 29 ml of glacial acetic acid, while maintaining a
temperature of 20°C. The 1-(o-chlorophenyl)-2-methyl-2-propanol is added
moderately fast, allowing the temperature to rise spontaneously. After
completing the addition, the reaction mixture is heated to 70°C and stirred,
and is then poured onto a mixture of water and ice. The aqueous mixture is
neutralized with sodium carbonate and extracted with diethyl ether. The
organic solution is washed with water, dried over magnesium sulfate and
evaporated to dryness.
The oily residue is taken up in 100 ml of 6 N aqueous hydrochloric acid and
refluxed until a clear solution is obtained. The latter is made basic with
aqueous ammonia and extracted with diethyl ether; the organic solution is
separated, washed, dried and evaporated. The residue is distilled under
reduced pressure to yield 26.3 g of 1-(o-chlorophenyl)-2-methyl-2-
propylamine, BP 116° to 118°C/16 mm.
The 1-(o-chlorophenyl)-2-methyl-2-propylamine hydrochloride is prepared by
adding ethanolic hydrogen chloride to an ice-cold solution of the free base in
ethanol; the desired salt precipitates and is recrystallized from ethanol, MP
245° to 246°C.
Therapeutic Function
Antiobesity
Properties of Clortermine
| Boiling point: | bp16 116-118° |
| Density | 1.074±0.06 g/cm3(Predicted) |
| pka | 9.60±0.25(Predicted) |
Safety information for Clortermine
Computed Descriptors for Clortermine
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran





You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4