Clomazone
- CAS NO.:81777-89-1
- Empirical Formula: C12H14ClNO2
- Molecular Weight: 239.7
- MDL number: MFCD00072457
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
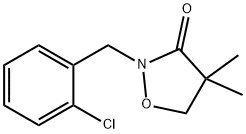
What is Clomazone?
Description
The molecular target site of clomazone has recently been determined. With clomazone, carotenoid synthesis is inhibited, but no intermediates in the carotenoidcommitted portion of the pathway accumulate (5,6). Synthesis of the derivatives of GGPP (gibberellic acid, phytol, carotenoids) is inhibited by clomazone (5–8). However, the synthesis of certain sesquiterpenoids and triterpenoids is not inhibited (9). Until recently, there was no credible report of the effect of clomazone on any enzyme of the terpenoid pathway (10–12). This was due to the fact that clomazone is a proherbicide and that the proper enzyme had not been tested.
Chemical properties
Depending purity, it may be clear and colorless to pale yellow or brownish liquid. Commercially available as emulsifiable concentrates that can be dissolved in water
The Uses of Clomazone
Herbicide.
Definition
ChEBI: A oxazolidinone that is 1,2-oxazolidin-3-one substituted by a 2-chlorobenzyl group at position 2 and two methyl groups at position 4.
Hazard
Moderately toxic by ingestion, inhalation, and skin contact. A reproductive hazard.
Agricultural Uses
Herbicide: Clomazone is a broad-spectrum herbicide used on rice, peas, pumpkins, soybeans, sweet potatoes, winter squash, cotton, tobacco and fallow wheat fields to control annual grasses and broadleaf weeds.
Trade name
CERANO®; COLZOR TRIO®; COMMAND®; COMMENCE®, DIBEL®; FMC® 57020; GAMBIT®; MAGISTER®; MERIT®; STRATEGY®
Potential Exposure
Clomazone is a oxazolidione broadspectrum herbicide used on rice, peas, pumpkins, soybeans, sweet potatoes, winter squash, cotton, tobacco, and fallow wheat fields to control annual grasses and broadleaf weeds.
Metabolic pathway
By the preparative incubation of clomazone with microorganisms that have the ability to metabolize clomazone, the metabolites are identified via major biotransformation reactions which involve hydroxylation at the 5-methylene carbon and one of the 3-methyl groups of the isoxazolidone ring and at the 3 0 -position of the phenyl ring. Minor metabolic routes include dihydroxylation on the phenyl ring, cleavage of the isoxazolinone ring, or complete removal of the isoxazolinone ring to form 2-chlorobenzyl alcohol. Under aerobic conditions of soils, degradation of clomazone proceeds primarily by CO2 evolution and the formation of bound soil residues. In flooded soils, clomazone is found rapidly to degrade via the formation of the reductive product N-[(2-chlorophenyl)methyl]-3-hydroxy-2,2- dimethylpropionamide. In tolerant soybean cell suspension cultures, the only metabolite identified is b-glycosyl-2-chlorobenzyl alcohol.
Shipping
UN2902 Pesticide, liquid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Do not discharge into drains or sewers. Dispose of waste material as hazardous waste using a licensed disposal contractor to an approved landfill. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of Clomazone
| Melting point: | 25°C |
| Boiling point: | 275.4°C |
| Density | 1.192 |
| refractive index | 1.5388 (estimate) |
| Flash point: | 157 °C |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMF: 33 mg/ml,DMSO: 16 mg/ml,Ethanol: 33 mg/ml,PBS (pH 7.2): 1 mg/ml |
| form | neat |
| pka | -1.48±0.40(Predicted) |
| form | Solid |
| color | White to off-white |
| Water Solubility | 1.101g/L(temperature not stated) |
| BRN | 7480026 |
| CAS DataBase Reference | 81777-89-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Dimethazone(81777-89-1) |
| EPA Substance Registry System | Clomazone (81777-89-1) |
Safety information for Clomazone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Clomazone
| InChIKey | KIEDNEWSYUYDSN-UHFFFAOYSA-N |
Clomazone manufacturer
LANSPHARMA
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
 81777-89-1 2-(2-chlorobenzyl)-4,4-dimethylisoxazolidin-3-one 98%View Details
81777-89-1 2-(2-chlorobenzyl)-4,4-dimethylisoxazolidin-3-one 98%View Details
81777-89-1 -
 Clomazone 95% CAS 81777-89-1View Details
Clomazone 95% CAS 81777-89-1View Details
81777-89-1 -
 Clomazone 98% (GC) CAS 81777-89-1View Details
Clomazone 98% (GC) CAS 81777-89-1View Details
81777-89-1 -
 Clomazone CAS 81777-89-1View Details
Clomazone CAS 81777-89-1View Details
81777-89-1 -
 Clomazone CAS 81777-89-1View Details
Clomazone CAS 81777-89-1View Details
81777-89-1 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
