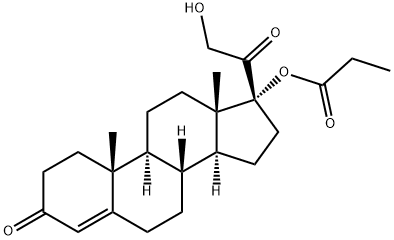
Clascoterone
- CAS NO.:19608-29-8
- Empirical Formula: C24H34O5
- Molecular Weight: 402.52
- MDL number: MFCD18384978
- EINECS: 685-282-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-05 19:05:58
What is Clascoterone?
Absorption
Upon topical application, clascoteronet permeates the skin to the dermal levels with minimal systemic absorption. In clinical trials, adult subjects with moderate to severe facial acne vulgaris received twice-daily topical application of six grams of clascoterone. The steady-state concentrations of the drug were reached within five days. Following two weeks, the mean ± SD Cmax was 4.5 ± 2.9 ng/mL and the mean ± SD area under the plasma concentration-time over the dosing interval (AUC?) was 37.1 ± 22.3 h*ng/mL. The mean ± SD average plasma concentration (Cavg) was 3.1 ± 1.9 ng/mL.
Toxicity
There is no information available on the toxicity profile of clascoterone, such as LD50 and overdose in humans.
Description
Clascoterone (Winlevi®) is an androgen receptor inhibitor being developed as a topical cream and solution by Cassiopea (a spin-out company of Cosmo Pharmaceuticals) for the treatment of androgen-dependent skin disorders, including androgenetic alopecia and acne vulgaris. Although the exact mechanism of action of clascoterone for the topical treatment of acne vulgaris is unknown, the drug is believed to compete with the androgen dihydrotestosterone for binding to androgen receptors in the sebaceous gland and hair follicles to attenuate signalling necessary for acne pathogenesis. In August 2020, clascoterone cream 1% received its first approval in the USA for the topical treatment of acne vulgaris in patients 12 years of age or older. Clinical studies of a different formulation of clascoterone (a solution containing a higher concentration of the drug) for the treatment of androgenetic alopecia are underway in Germany and the USA. This article summarizes the milestones in the development of clascoterone leading to this first approval for the topical treatment of acne vulgaris.
Chemical properties
Clascoterone is a white to almost white powder, practically insoluble in water. The compound has the empirical formula C24H34O5 andmolecular weight of 402.5 g/mol.
The Uses of Clascoterone
Clascoterone (cortexolone 17α-propionate, CB-03-01) is a novel antagonist of androgen receptors. It binds to androgen receptors with high affinity. By competing with androgens for binding to androgen receptors, clascoterone works by blocking the androgen receptor signalling cascades that promote acne pathogenesis, such as sebaceous gland proliferation, excess sebum production, and inflammatory pathways. In August 2020, FDA approved clascoterone for the first-in-class topical treatment of acne (acne vulgaris) in male and female patients 12 years and older. Clascoterone is also being investigated as a novel treatment for androgenetic alopecia.
The Uses of Clascoterone
Clascoterone is being developed as a topical cream by Cassiopea for androgen-?dependent skin disorders.
Background
Clascoterone (cortexolone 17α-propionate, CB-03-01) is a novel antagonist of androgen receptors. It binds to androgen receptors with high affinity. By competing with androgens for binding to androgen receptors, clascoterone works by blocking the androgen receptor signalling cascades that promote acne pathogenesis, such as sebaceous gland proliferation, excess sebum production, and inflammatory pathways. In August 2020, FDA approved clascoterone for the first-in-class topical treatment of acne (acne vulgaris) in male and female patients 12 years and older. Clascoterone is also being investigated as a novel treatment for androgenetic alopecia.
Indications
Clascoterone is indicated for the topical treatment of acne vulgaris in patients 12 years of age and older.
Biological Activity
Clascoterone is a high-affinity androgen receptor (AR) antagonist (antiandrogen) that antagonizes testosterone-stimulated transcription activity (in reporter cells) as well as DHT-induced production of lipids and inflammatory cytokines (in human sebocytes) in cultures. When applied topically, clascoterone is as active as cyproterone acetate, and 2-, 3-, and 4-times, respectively, more active than finasteride, flutamide, and progesterone in vivo (local antiandrogenic activity in hamster′s flank organ; 100-400 μg/50 μL acetone/animal).
Pharmacokinetics
Clascoterone exerts anti-androgenic effects by working as an antagonist at androgen receptors (ARs) expressed throughout the skin, including sebaceous glands, sebocytes, and dermal papilla cells. Clascoterone blocks the effects of testosterone and dihydrotestosterone (DHT), which are androgens that bind to the ARs and contribute to the development of androgen-dependent conditions such as acne and alopecia. In vitro, the antiandrogenic effects of clascoterone in human primary sebocytes occurred in a dose-dependent manner. Clascoterone mediates selective topical activity by mainly targeting androgen receptors at the site of application. It has limited systemic effects.
In clinical trials, HPA axis suppression was observed as a 30-minute post-stimulation serum cortisol level of ≤18 mcg/dL in 5% of adult subjects and 9% of adolescent subjects with acne vulgaris following two weeks of topical treatment of clascoterone. HPA axis function returned to normal following the discontinuation of drug treatment.
Side Effects
Clascoterone topical application may cause serious side effects: severe itching, burning, peeling, or redness of treated skin; high blood potassium--nausea, weakness, tingly feeling, chest pain, irregular heartbeats, loss of movement; or decreased adrenal gland hormones--nausea, vomiting, stomach pain, loss of appetite, feeling tired or light-headed, muscle or joint pain, skin discoloration, craving salty foods.
Safety
Clascoterone(Winlevi) is the only topical acne product with a warning about hypothalamic-pituitary-adrenal (HPA) axis suppression. This may result when Winlevi is used over large surface areas or if use is prolonged. In addition, pediatric patients may be more susceptible. This adverse event was not observed in the pivotal studies or in the long-term open-label extension study. However, it was observed in a small group of patients on Day 14 in a pharmacokinetic study. Normal HPA axis function was observed at follow-up at 4 weeks after end of treatment.
Metabolism
According to in vitro and clinical studies, the main possible primary metabolite of clascoterone is cortexolone, which is an inactive metabolite. The plasma concentrations of cortexolone were generally below or near the lower limit of quantitation (0.5 ng/mL). Although clascoterone penetrates the skin, the systemic activity of the drug is limited due to rapid hydrolysis of clascoterone into the inactive metabolite by skin and plasma esterases, namely carboxylesterase.
Mode of action
Clascoterone is an androgen receptor inhibitor. The mechanism of action of WINLEVI cream for the topical treatment of acne vulgaris is unknown.
Properties of Clascoterone
| Boiling point: | 538.9±50.0 °C(Predicted) |
| Density | 1.19 |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | Powder |
| pka | 12.98±0.10(Predicted) |
| color | White to light yellow |
Safety information for Clascoterone
Computed Descriptors for Clascoterone
| InChIKey | GPNHMOZDMYNCPO-PDUMRIMRSA-N |
| SMILES | C1(=O)C=C2[C@](C)(CC1)[C@]1([H])[C@]([H])([C@@]3([H])[C@@](CC1)(C)[C@@](OC(=O)CC)(C(=O)CO)CC3)CC2 |
Abamectin manufacturer
Symbiotec Pharma Lab Pvt Ltd
Related products of tetrahydrofuran
You may like
-
 19608-29-8 Clascoterone API 98%View Details
19608-29-8 Clascoterone API 98%View Details
19608-29-8 -
 19608-29-8 98%View Details
19608-29-8 98%View Details
19608-29-8 -
 Clascoterone 98%View Details
Clascoterone 98%View Details
19608-29-8 -
 Clascoterone CAS 19608-29-8View Details
Clascoterone CAS 19608-29-8View Details
19608-29-8 -
 77-92-9 99%View Details
77-92-9 99%View Details
77-92-9 -
 77-92-9 99%View Details
77-92-9 99%View Details
77-92-9 -
 8000-27-9 CEDAR WOOD OIL 99%View Details
8000-27-9 CEDAR WOOD OIL 99%View Details
8000-27-9 -
 95-54-5 99%View Details
95-54-5 99%View Details
95-54-5