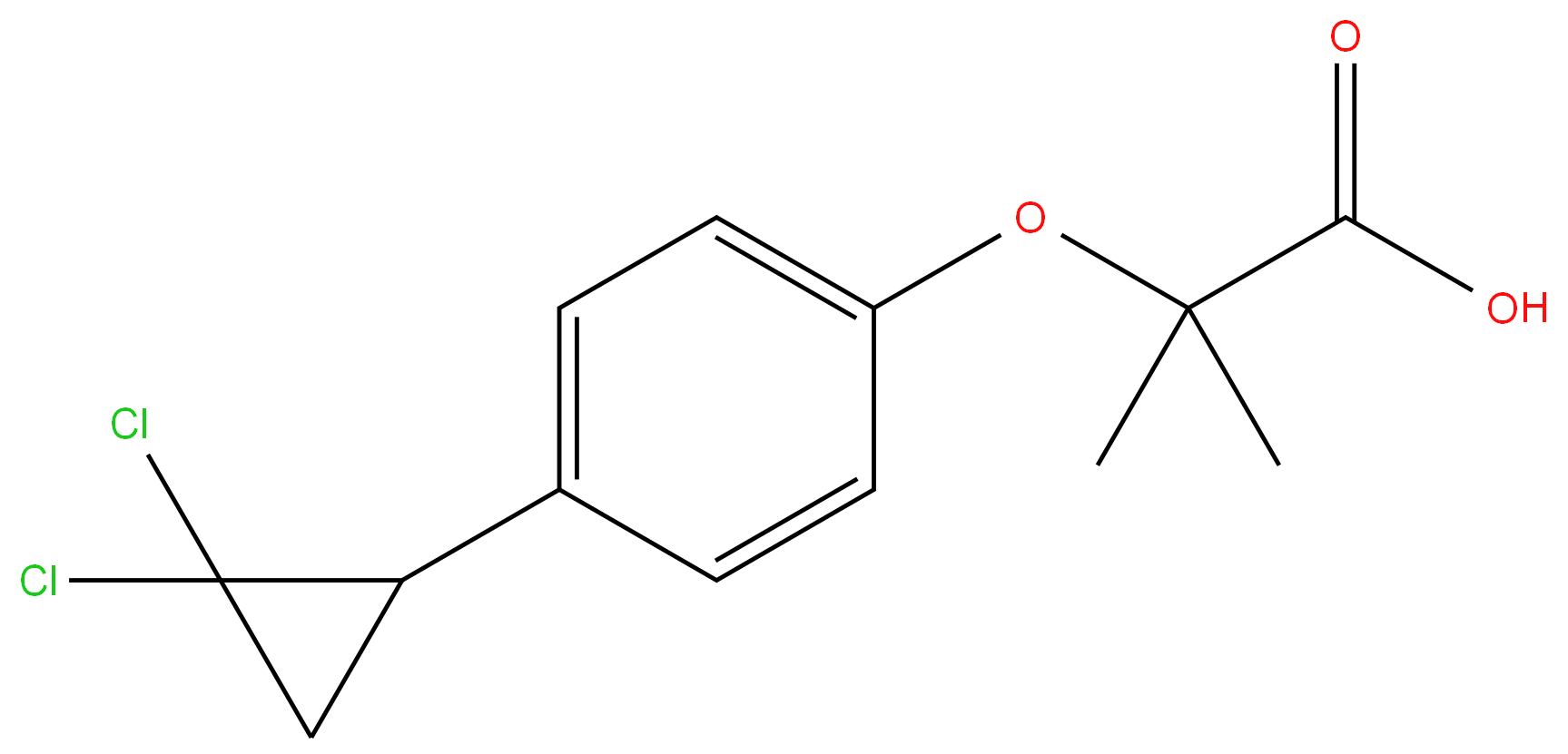
Ciprofibrate
Synonym(s):2-[p-(2,2-Dichlorocyclopropyl)phenoxy]-2-methylpropanoic acid
- CAS NO.:52214-84-3
- Empirical Formula: C13H14Cl2O3
- Molecular Weight: 289.15
- MDL number: MFCD00467135
- EINECS: 257-744-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16

What is Ciprofibrate?
Description
Ciprofibrate is a potent, long-acting hypolipidemic agent related to clofibrate, bezafibrate and fenofibrate. It is effective in types IIa, IIb, IIX and IV hyperlipoproteinemias, and produces a beneficial elevation of the anti-atherogenic HDL.
Description
Ciprofibrate is an agonist of peroxisome proliferator-activated receptor α (PPARα; EC50 = 0.9 μM in a transactivation assay). It is selective for PPARα over PPARγ and PPARδ at 300 μM. Ciprofibrate (250 μM) induces cell cycle arrest at the G2/M and S phases in Fao rat, but not HepG2 human, hepatocellular carcinoma cells. It decreases fasting plasma levels of triglycerides and increases fasting plasma glucose levels in the apolipoprotein CIII transgenic mouse model of hypertriglyceridemia when administered at a dose of 10 mg/kg. Formulations containing ciprofibrate have been used in the treatment of hypertriglyceridemia.
Chemical properties
Off-White to Pale Beige Solid
Originator
Sterling-Wintbrop (USA)
The Uses of Ciprofibrate
Peroxisome proliferator-activated receptor α (PPARα) is a ligand-activated transcription factor involved in the regulation of lipid homeostasis. Activation of PPARα results in expression of a variety of genes, particularly those involved in fatty acid β-oxidation, binding, and transport. Ciprofibrate activates PPARα with an EC50 value of 20 μM and only marginally affects PPARγ (EC50 = >300 μM). It has been shown to lower adipose tissue weight and reduce plasma insulin concentrations in obese rats and has been used clinically in the treatment of dyslipidemia. Ciprofibrate reportedly stimulates cholesteryl ester transfer protein expression and improves the flow of cholesterol through the indirect reverse cholesterol transport system, preserving plasma HDL.
The Uses of Ciprofibrate
antihyperlipidemic
The Uses of Ciprofibrate
Ciprofibrate is a hypolipemic agent, related structurally to Clofibrate (C586910). Ciprofibrate is used as an antilipemic.
What are the applications of Application
Ciprofibrate is a hypolipidemic agonist of PPARα
Definition
ChEBI: Ciprofibrate is a monocarboxylic acid, a member of cyclopropanes and an organochlorine compound. It has a role as an antilipemic drug.
Manufacturing Process
A mixture of 8 g (0.0356 mol) of p-(2,2-dichlorocyclopropyl)phenol, 11.2 g (0.28 mol) of sodium hydroxide pellets, 11 g of chloroform and 350 ml of acetone was prepared at 0°C. The cooling bath was removed, the mixture stirred for a minute and then heated on a steam bath to reflux temperature. The reaction mixture was stirred at reflux for three hours and then concentrated in vacuo. The residual gum was partitioned between dilutehydrochloric acid and ether, and the ether layer was separated, dried and concentrated in vacuo. The residual oil (14 g) was partitioned between dilute aqueous sodium bicarbonate and ether. The sodium bicarbonate solution was acidified with concentrated hydrochloric acid and extracted with ether. The ether solution was dried over anhydrous sodium sulfate and concentrated. The residue (9.5 g of yellow oil) was crystallized twice from hexane to give 6.0 g of 2-[p-(2,2-dichlorocyclopropyl)phenoxy]-2-methyl propionic acid in the form of a pale cream-colored solid, MP 114°C to 116°C.
brand name
LJPANOR
Therapeutic Function
Antihyperlipidemic
World Health Organization (WHO)
The safety profile of ciprofibrate is similar to that of clofibrate. See also under clofibrate in full edition.
Biochem/physiol Actions
Peroxisome proliferator-activated receptor α (PPARα) agonist
Clinical Use
Hyperlipidaemia
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: increased risk of myopathy with
daptomycin - try to avoid concomitant use.
Anticoagulants: enhances effect of coumarins and
phenindione. Dose of anticoagulant should be
reduced by up to 50% and readjusted by monitoring
INR.
Antidiabetics: may improve glucose tolerance
and have an additive effect with insulin or
sulphonylureas.
Colchicine: possible increased risk of myopathy.
Lipid-regulating drugs: increased risk of myopathy
in combination with statins and ezetimibe (Do
not exceed 10 mg of simvastatin and 20 mg of
rosuvastatin.1
) - avoid with ezetimibe.
Metabolism
Approximately 30-75% of a single dose administered to volunteers was excreted in the urine in 72 hours, either as unchanged ciprofibrate (20-25% of the total excreted) or as a glucuronide conjugate. Subjects with moderate renal impairment excreted on average 7% of a single dose as unchanged ciprofibrate over 96 hours, compared with 6.9% in normal subjects. In subjects with severe insufficiency this was reduced to 4.7%.
Metabolism
Not Available
Properties of Ciprofibrate
| Melting point: | 114-116° |
| Boiling point: | 401.74°C (rough estimate) |
| Density | 1.2576 (rough estimate) |
| refractive index | 1.5209 (estimate) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, freely soluble in anhydrous ethanol, soluble in toluene. |
| form | neat |
| pka | 3.31±0.10(Predicted) |
| form | Solid |
| color | White to Pale Beige |
| Merck | 14,2313 |
| CAS DataBase Reference | 52214-84-3(CAS DataBase Reference) |
| EPA Substance Registry System | Propanoic acid, 2-[4-(2,2-dichlorocyclopropyl)phenoxy]-2-methyl- (52214-84-3) |
Safety information for Ciprofibrate
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H350:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Ciprofibrate
| InChIKey | KPSRODZRAIWAKH-UHFFFAOYSA-N |
Ciprofibrate manufacturer
Sai Life Sciences Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran




You may like
-
 52214-84-3 Ciprofibrate 98%View Details
52214-84-3 Ciprofibrate 98%View Details
52214-84-3 -
 52214-84-3 99%View Details
52214-84-3 99%View Details
52214-84-3 -
 Ciprofibrate 98%View Details
Ciprofibrate 98%View Details
52214-84-3 -
 Ciprofibrate 52214-84-3 98%View Details
Ciprofibrate 52214-84-3 98%View Details
52214-84-3 -
 Ciprofibrate CAS 52214-84-3View Details
Ciprofibrate CAS 52214-84-3View Details
52214-84-3 -
 Ciprofibrate CAS 52214-84-3View Details
Ciprofibrate CAS 52214-84-3View Details
52214-84-3 -
 Ciprofibrate CAS 52214-84-3View Details
Ciprofibrate CAS 52214-84-3View Details
52214-84-3 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8