CHLOROSILANE
- CAS NO.:13465-78-6
- Empirical Formula: ClH3Si
- Molecular Weight: 66.56
- MDL number: MFCD07777018
- EINECS: 236-705-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-08 15:51:14
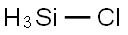
What is CHLOROSILANE?
Chemical properties
colorless gas; enthalpy of vaporization 21kJ/mol; entropy of vaporization 82.8kJ/(mol·K) [CIC73] [CRC10]
General Description
Chlorosilanes are a class of liquids that range in color from clear colorless to light yellow colored. A clear to light yellow liquid. Slightly more dense than water. Contact may burn skin, eyes and mucous membranes. May be toxic by ingestion, inhalation, and skin absorption. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Based on the properties of similar materials, there is the possibility that the reaction of CHLOROSILANE with water may be vigorous or violent. Products of the reaction include hydrogen chloride. The reaction generates heat and this heat may be sufficient to ignite the product. The chlorosilicon hydrides(ClxSiHy) are spontaneously flammable in air, NFPA 1991.
Reactivity Profile
Chlorosilanes are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases.
Health Hazard
Highly toxic: contact with water produces toxic gas, may be fatal if inhaled. Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Produce flammable and toxic gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Containers may explode when heated. Runoff may create fire or explosion hazard.
Properties of CHLOROSILANE
| Melting point: | -118°C |
| Boiling point: | -30,4°C |
| Density | 1,145 g/cm3 |
| Flash point: | -90°C (-133°F) |
| form | colorless gas |
| Specific Gravity | 1.145 |
| color | colorless |
| Hydrolytic Sensitivity | 10: reacts extremely rapidly with moisture and oxygen - may be pyrophoric - sealed system required |
| EPA Substance Registry System | Silane, chloro- (13465-78-6) |
Safety information for CHLOROSILANE
Computed Descriptors for CHLOROSILANE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4