ALLYLTRICHLOROSILANE
- CAS NO.:107-37-9
- Empirical Formula: C3H5Cl3Si
- Molecular Weight: 175.52
- MDL number: MFCD00000486
- EINECS: 203-485-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
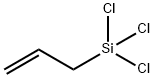
What is ALLYLTRICHLOROSILANE?
Chemical properties
Clear colorless to slightly yellow liquid
Chemical properties
Allyl trichhlorosilane is a volatile, corrosive, flammable, colorless liquid. Irritating odor.
Physical properties
bp 116°C/750 Torr; nD 1.4450; d 1.211.
The Uses of ALLYLTRICHLOROSILANE
Allyltrichlorosilane acts as a reagent that produces homoallylic alcohols upon reaction with aldehydes in DMF. It is used to make silicones and glass fiber fininshes.
The Uses of ALLYLTRICHLOROSILANE
Intermediate for silicones, fiberous glass finishes.
What are the applications of Application
Allyltrichlorosilane is a reagent that produces homoallylic alcohols upon reaction with aldehydes in DMF
Preparation
allyl trichlorosilane is conveniently prepared from allyl chloride, Cl3SiH, CuCl, and (i-Pr)2EtN (or Et3N) in ether at 20°C,or from allyl chloride and coppersilicon powder at 250°C.Other methods include the reaction of allyl chloride with SiCl4 in the presence of CuCl and Et3N7 or in the presence of Cp2Ni and HMPA at 90°C,the flash pyrolysis of allyl chloride with Si2Cl2 in PhCl at 500°C,the reaction of allylMgBr with SiCl4,coupling of allene with Cl3SiH, catalyzed by (Ph3P)4Pd (120°C, 5 h),11 and the elimination of HCl from Cl(CH2)3SiCl3 using, e.g., quinoline as base.The analogous (E)-crotyl trichlorosilane can be synthesized from (E)-crotyl chloride, Cl3SiH, CuCl, and (i-Pr)2EtN in ether at 20°C,or from (E)-crotyl chloride, SiCl4, and Cp2Ni in HMPA at 90°C.The reaction of (E)-crotyl chloride with Cl3SiH and Bu4PCl at 150°C has also been reported. (Z)-crotyl trichlorosilane, on the other hand, was prepared via the 1,4-addition of Cl3SiH to butadiene, catalyzed by (Ph4P)4Pd (at 20°Cor?78°C)or by (PhCN)2PdCl2;this hydrosilylation can also be catalyzed by Nior proceed uncatalyzed at 500–600°C.Analogous trifluoro and tribromo derivatives C3H5SiX3 (X = F and Br) have also been described.
General Description
A colorless liquid with a pungent odor. Flash point of 95°F. Corrosive to metals and tissue.
Reactivity Profile
Chlorosilanes, such as ALLYLTRICHLOROSILANE, are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases.
Health Hazard
Inhalation of vapor irritates mucous membranes. Liquid causes severe burns of eyes and skin and severe internal burns if ingested.
Safety Profile
Poison by intravenous route. Corrosive. See ALLYL COMPOUNDS. When heated to decomposition it emits toxic Cl-. A dangerous fire hazard. To fight fire, use foam, mist, spray, dry chemical.
Potential Exposure
Used to make silicones and glass fiber finishes.
Shipping
UN1724 Allyltrichlorosilane, stabilized, Hazard class: 8; Labels: 8-Corrosive material, 3-Flammable liquid
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Chlorosilanes react vigorously with bases and both organic and inorganic acids generating toxic and/or flammable gases. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride gas. They may also produce flammable gaseous hydrogen. Attacks metals in the presence of moisture. Avoid all sources of ignition.
Properties of ALLYLTRICHLOROSILANE
| Melting point: | 35℃ |
| Boiling point: | 116 °C750 mm Hg(lit.) |
| Density | 1.211 g/mL at 25 °C(lit.) |
| vapor density | >1 (vs air) |
| vapor pressure | 10 mm Hg ( 16.1 °C) |
| refractive index | n |
| Flash point: | 88 °F |
| storage temp. | Flammables area |
| solubility | soluble in MeCN, THF, CH2Cl2, toluene, benzene,
etc.; not compatible with protic solvents, acetone, and other
ketones. Dipolar aprotic solvents (DMF, DMSO, HMPA, etc.)
coordinate Si, thereby increasing the reactivity of C3H5SiCl3
with electrophiles. |
| form | Liquid |
| color | Clear colorless to slightly yellow |
| Specific Gravity | 1.211 |
| Water Solubility | Soluble in THF, toluene, benzene. Reacts with water. |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 1743449 |
| CAS DataBase Reference | 107-37-9(CAS DataBase Reference) |
| EPA Substance Registry System | Silane, trichloro-2-propen-1-yl- (107-37-9) |
Safety information for ALLYLTRICHLOROSILANE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H225:Flammable liquids H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for ALLYLTRICHLOROSILANE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Allyltrichlorosilane CAS 107-37-9View Details
Allyltrichlorosilane CAS 107-37-9View Details
107-37-9 -
 Allyltrichlorosilane CAS 107-37-9View Details
Allyltrichlorosilane CAS 107-37-9View Details
107-37-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1