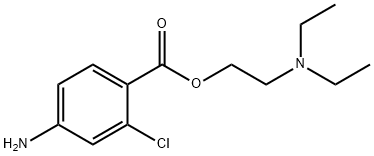
Chloroprocaine hydrochloride
Synonym(s):2-(Diethylamino)ethyl 4-amino-2-chlorobenzoate monohydrochloride
- CAS NO.:3858-89-7
- Empirical Formula: C13H20Cl2N2O2
- Molecular Weight: 307.2161
- MDL number: MFCD00025215
- EINECS: 223-371-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
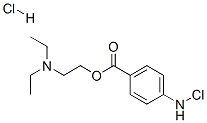
What is Chloroprocaine hydrochloride?
Chemical properties
crystalline solid
Originator
Nesacaine,Astra,US,1956
The Uses of Chloroprocaine hydrochloride
Chloroprocaine Hydrochloride is a local anaesthetic compound showing similar effects to Lidocaine.
The Uses of Chloroprocaine hydrochloride
Anesthetic (local).
Definition
ChEBI: The monohydrochloride salt of chloroprocaine. Used as a local anaesthetic, particularly for oral surgery, it has the advantage over lidocaine of constricting blood vessels, so reducing bleeding.
Manufacturing Process
In the first step, 2-chloro-4-aminobenzoyl chloride hydrochloride is prepared
by refluxing a mixture of 25 cc of purified thionyl chloride and 10 g of 2-
chloro-4-aminobenzoic acid until all of the solid has gone into solution. To the
cooled solution is added 150 cc of dry ethyl ether. A brisk stream of dry
hydrogen chloride is passed into the solution until the precipitation of 2-
chloro-4-aminobenzoylchloride hydrochloride is complete. The acyl halide is
removed by filtration and dried in a vacuum desiccator.
In the second step, the diethylaminoethyl 2-chloro-4-aminobenzoate
hydrochloride is prepared by refluxing equimolar proportions of the
hydrochloride of beta-diethylaminoethanol in a suitable inert solvent such as a
mixture of dry toluene and tetrachloroethane and the hydrochloride of 2-
chloro-4-aminobenzoyl chloride until the reaction as indicated by the cessation
of hydrogen chloride evolution is complete. The supernatant solvents are
decanted from the reaction product which can be conveniently purified by
crystallization from absolute ethanol.
An alternative purification can be effected by dissolving the reaction product in
water. The ester base is liberated by rendering the clarified aqueous solution
alkaline. Removal of the base from the alkaline solution is achieved by
extraction with a suitable solvent such as benzene or ether. The pure
hydrochloride of diethylaminoethyl 2-chloro-4-aminobenzoate is then
precipitated from the dried extract by the addition of dry hydrogen chloride.
After removal by filtration and recrystallization from ethanol it is found to
have a melting point of 173° to 174°C.
brand name
Nesacaine (Abraxis).
Therapeutic Function
Local anesthetic
Properties of Chloroprocaine hydrochloride
| Melting point: | 176-178° (microstage) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| InChI | InChI=1S/C13H19ClN2O2.ClH/c1-3-16(4-2)9-10-18-13(17)11-5-7-12(15-14)8-6-11;/h5-8,15H,3-4,9-10H2,1-2H3;1H |
Safety information for Chloroprocaine hydrochloride
Computed Descriptors for Chloroprocaine hydrochloride
| InChIKey | KSEXTRRPURJCDW-UHFFFAOYSA-N |
| SMILES | N(Cl)C1=CC=C(C(=O)OCCN(CC)CC)C=C1.[H]Cl |
Abamectin manufacturer
Surajlok Chemicals Pvt Ltd (Neon Laboratories Ltd)
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 3858-89-7 Chloroprocaine hydrochloride 98%View Details
3858-89-7 Chloroprocaine hydrochloride 98%View Details
3858-89-7 -
 Chloroprocaine hydrochloride CAS 3858-89-7View Details
Chloroprocaine hydrochloride CAS 3858-89-7View Details
3858-89-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4