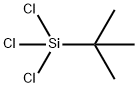
Chloromethyl silane
- CAS NO.:993-00-0
- Empirical Formula: CH5ClSi
- Molecular Weight: 80.59
- EINECS: 213-600-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
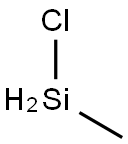
What is Chloromethyl silane?
Description
MethyI chlorosilane iS a colorless liquid. Flashpoint=-9"C. NJDHSS (New Jersey Department of Health and Senior Hazard Rating: Health 3 Flammability 4, Reactivity 2W. Reacts with water.Potential Exposure: MethyI chlorosilane is rarely used as a raw material, but it is released during the manufacture of Silicones or siloxanes.
Definition
One of several intermediates in the formation of silicones or siloxanes, they react with hydroxyl groups on many types of surfaces to produce a permanent, thin-surface film of silicone that imparts water repellency. Examples are methyltrichlorosilane, dimethyldichlorosilane, and trimethylchlorosilane.
General Description
Methyl chlorosilane is a colorless gas with a distinctive odor. Chloromethyl silane is insoluble in water. Its vapors are heavier than air. Contact with the material causes severe irritation to skin, eyes, and mucous membranes. Chloromethyl silane is toxic by ingestion and skin absorption. Chloromethyl silane is also a dangerous fire risk. Chloromethyl silane is used to make water repellent materials. Prolonged exposure of container to fire or intense heat may result in their violent rupturing and rocketing.
Air & Water Reactions
Highly flammable. Based on the properties of similar materials, there is the possibility that the reaction of Chloromethyl silane with water may be vigorous or violent. Products of the reaction include hydrogen chloride. The reaction generates heat and this heat may be sufficient to ignite the product. The chlorosilicon hydrides (ClxSiHy) are spontaneously flammable in air [NFPA 1991].
Reactivity Profile
Chlorosilanes, such as Chloromethyl silane, are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases.
Hazard
Toxic by ingestion and inhalation, strong irritant to skin and eyes.
Health Hazard
TOXIC; may be fatal if inhaled or absorbed through skin. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Flammable; may be ignited by heat, sparks or flames. May form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. Vapors may travel to source of ignition and flash back. Some of these materials may react violently with water. Cylinders exposed to fire may vent and release toxic and flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket. Runoff may create fire or explosion hazard.
Flammability and Explosibility
Extremely flammable
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least30 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, getmedical attention. If victim is conscious, administer wateror milk. Do not induce vomiting. Medical observation isrecommended for 24- - 48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmo-nary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
storage
Color Code—Red Stripe: Flammability Hazard:Store separately from all other flammable materials. Priorto working with this chemical you should be trained on itsproper handling and storage. Methyl chlorosilane must bestored to avoid contact with water, steam, and moisturebecause toxic and corrosive chloride gases, including hydrogen chloride, can be produced. Sources of ignition, such assmoking and open flames, are prohibited where methylchlorosilane is handled, used, or stored.
Shipping
Methylchlorosilane requires a shipping label of“POISON GAS, FLAMMABLE GAS, CORROSIVE.” Itfalls in Hazard Class 2.3. It is a violation of transportationregulations to refill compressed gas cylinders without theexpress written permission of the owner
Incompatibilities
May form explosive gases with air.Contact with water, steam, or moisture, forms toxic and corrosive hydrogen chloride gas. Not compatible with strongbases, strong acids, oxidizers. Attacks metals, plastics, rubbers, and coatings.
Properties of Chloromethyl silane
| Melting point: | -134.1°C |
| Boiling point: | 8.7°C (estimate) |
| Density | 0.8840 |
| form | liquid |
| EPA Substance Registry System | Methylchlorosilane (993-00-0) |
Safety information for Chloromethyl silane
Computed Descriptors for Chloromethyl silane
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
![2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
