Amyltrichlorosilane (mixed isomers)(Pentyltrichlorosilane)
- CAS NO.:107-72-2
- Empirical Formula: C5H11Cl3Si
- Molecular Weight: 205.59
- MDL number: MFCD00000487
- EINECS: 203-515-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
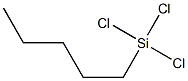
What is Amyltrichlorosilane (mixed isomers)(Pentyltrichlorosilane)?
Description
Amyl trichlorosilane is a colorless to yellowish liquid with a sharp acrid odor (like hydrochloric acid).Molecular weight= 205.63; Boiling point= 168℃;Specific gravity= 1.137 at 25℃; Liquid surface tension= 5(estimate) 0.020 N/m at 20℃; Relative vapor density(air= 1) 5 7.1; Latent heat of vaporization= 2.02 3 105J/kg; Heat of combustion 5 2 154 3 105 J/kg; Heat ofsolution: 4.0 3 105 J/kg; Flash point= 30℃. NFPA 704 MHazard Identification (based on NFPA-704 M RatingSystem): Health 3, Flammability 2, Reactivity 2 . Reactswith water.
Chemical properties
Colorless to yellow liquid. Readily hydrolyzed by moisture with the liberation of hydrogen chloride. Combustible.
Chemical properties
Amyl trichlorosilane is a colorless to yellowish liquid with a sharp acrid odor (like hydrochloric acid).
The Uses of Amyltrichlorosilane (mixed isomers)(Pentyltrichlorosilane)
Intermediate for silicones.
General Description
A colorless to yellow liquid with a pungent odor. Amyltrichlorosilane (mixed isomers)(Pentyltrichlorosilane) is combustible and has a flash point of 145°F. Corrosive to metals and tissue.
Reactivity Profile
Chlorosilanes, such as Amyltrichlorosilane (mixed isomers)(Pentyltrichlorosilane), are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases.
Hazard
Toxic and corrosive.
Health Hazard
Inhalation causes irritation of mucous membrane. Contact of liquid with eyes or skin causes severe burns, and ingestion causes severe burns of mouth and stomach.
Safety Profile
Moderately toxic by ingestion and skin contact. Mildly toxic by inhalation. A corrosive irritant to the eyes, skin, and mucous membranes. When heated to decomposition it emits toxic fumes of Cl-. See also CHLOROSILANES
Potential Exposure
It is used to make silicones.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 24°48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
storage
Color Code—White: Corrosive or ContactHazard; Store separately in a corrosion-resistant location.Prior to working with this chemical you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area. Metal containersinvolving the transfer of this chemical should be groundedand bonded. Where possible, automatically pump liquidfrom drums or other storage containers to process containers. Drums must be equipped with self-closing valves,pressure vacuum bungs, and flame arresters. Use only nonsparking tools and equipment, especially when openingand closing containers of this chemical. Sources of ignition, such as smoking and open flames, are prohibitedwhere this chemical is used, handled, or stored in a mannerthat could create a potential fire or explosion hazard.
Shipping
UN1728 Amyltrichlorosilane, Hazard class: 8; Labels: 8-Corrosive material.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Chlorosilanes react vigorously with bases and both organic and inorganic acids generating toxic and/or flammable gases. Chlorosilanes react with water, moist air, or steam releasing heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous hydrogen. Attacks metals in the presence of moisture.
Properties of Amyltrichlorosilane (mixed isomers)(Pentyltrichlorosilane)
| Boiling point: | 173 °C760 mm Hg(lit.) |
| Density | 1.142 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 30°C |
| form | A liquid |
| Specific Gravity | 1.14 |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| EPA Substance Registry System | Silane, trichloropentyl- (107-72-2) |
Safety information for Amyltrichlorosilane (mixed isomers)(Pentyltrichlorosilane)
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H226:Flammable liquids H311:Acute toxicity,dermal H314:Skin corrosion/irritation H331:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Amyltrichlorosilane (mixed isomers)(Pentyltrichlorosilane)
New Products
4-Piperidinemethanol Ethyl 2,4-Dihydroxy-6-methylnicotinate Ethyl isonicotinate 3-pyridine methanol N-Methyl 4-chloro-pyridine-2-carboxamide 2-Fluoro-6-iodobenzoic acid 2-((2,6-difluorobenzyl)(ethoxycarbonyl)amino)-4-((dimethylamino)methyl)-5-(4-nitrophenyl)thiophene-3-carboxylic acid Ethyl2-oxo-2,3,9,10-tetrahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline-8(7H)-carboxylate Elinzanetant tert-butyl 2-(4-amino-6-chloropyrimidin-5-yloxy)ethylmethylcarbamate Phenylazomalononitrile 5,6 Dimethoxy-1-indanone 3-Iodophenylacetic acid 2-Hexyn-1-ol Dibenzo-18-crown-6 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole N Ethylmethylamine Ethyl Methanesulfonate N N' DimethylEthylenediamine Lead II Bromide Variamine Blue B Diazonium salt N N N'Trimethyl ethylenediamineRelated products of tetrahydrofuran



You may like
-
![4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%View Details
4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%View Details
3680-69-1 -
 4'-Benzyloxy-2-bromopropiophenone 98%View Details
4'-Benzyloxy-2-bromopropiophenone 98%View Details
35081-45-9 -
 53928-30-6 98%View Details
53928-30-6 98%View Details
53928-30-6 -
 5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 -
 4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
79307-93-0 -
 Quinoline-8-sulfonyl Chloride 98%View Details
Quinoline-8-sulfonyl Chloride 98%View Details
18704-37-5 -
 29676-71-9 99%View Details
29676-71-9 99%View Details
29676-71-9 -
 (R)-2-amino-N-benzyl-3-methoxypropanamide 98%View Details
(R)-2-amino-N-benzyl-3-methoxypropanamide 98%View Details
196601-69-1
