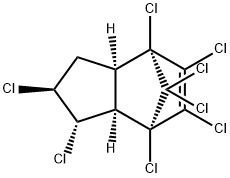
CHLORDANE
- CAS NO.:57-74-9
- Empirical Formula: C10H6Cl8
- Molecular Weight: 409.78
- MDL number: MFCD00072502
- EINECS: 200-349-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
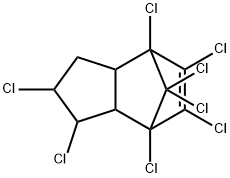
What is CHLORDANE?
Description
Chlordane is a viscous, amber-coloured liquid. Technical-grade chlordane is a mixture of many structurally related compounds including trans-chlordane, cis-chlordane, chlordene, heptachlor, and trans-nonachlor. Chlordane was used as a broad-spectrum pesticide in the United States from 1948 to 1988. The uses included termite control in homes and pest control on agricultural crops such as maize and citrus, on home lawns, gardens, turf, and ornamental plants. Chlordane is a persistent organochlorine insecticide. It kills insects when ingested and on contact. Formulations include dusts, emulsifiable concentrates, granules, oil solutions, and wettable powder.
Chemical properties
Chlordane is a viscous, amber-colored liquid. Technical grade chlordane is a mixture of many structurally related compounds including trans-chlordane, cis-chlordane, -chlordene, heptachlor, and trans-nonachlor. 14,15 Chlordane was used as a broad-spectrum pesticide in the United States from 1948 to 1988. Its uses included termite control in homes; pest control on agricultural crops such as maize and citrus, on home lawns, gardens, turf, and ornamental plants. Chlordane is a persistent organochlorine insecticide. It kills insects when ingested and on contact. Formulations include dusts, emulsifi able concentrates, granules, oil solutions, and wettable powder.
Chemical properties
off-white powder
Chemical properties
Chlordane is a colorless, or light-yellow or amber, thick liquid. Pungent, chlorine-like odor. It may occur as a crystalline solid.
Physical properties
Colorless to amber to yellowish-brown, viscous liquid. Technical formulations impart an aromatic, slight pungent odor similar to chlorine
The Uses of CHLORDANE
Insecticide and fumigant.
The Uses of CHLORDANE
Insecticide, fumigant.
The Uses of CHLORDANE
Chlordane is a persistent non-systemic contact and ingested insecticide with some fumigant action. It is used as a wood preservative, protective treatment for underground cables, and to reduce earthworm populations in lawns. It is used against Formicidae, Coleoptera, Noctuidae larva, Saltatoria, subterranean termites, and many other insect pests. It controls household insects, pests of man and domestic animals.
Definition
ChEBI: Chlordane is a cyclodiene organochlorine insecticide. It has a role as a GABA-gated chloride channel antagonist and a persistent organic pollutant. It derives from a hydride of an indene.
Hazard
A possible carcinogen. Toxic by ingestion, inhalation, and skin absorption. Liver damage.
Health Hazard
Highly toxic to humans by ingestion; moderately toxic in test animals; skin absorption or inhalation of its vapors can producepoisoning effects; exhibits acute, delayed,and chronic effects; symptoms include nausea, vomiting, abdominal pain, irritation,confusion ataxia, tremor, and convulsions;delayed development of liver disease andblood disorder also reported (U.S. EPA1988); human death may result from ingestion of 10–20 g of pure compound or topical skin application of 50 g in 30 minutes;moderately irritating to skin; oral LD50value in rats ~300 mg/kg; exposure limit0.5 mg/kg: exposure limit 0.5 mg/m3 (skin);RCRA Waste Number U036; US EPA listedextremely hazardous substance; LD50 data inliterature inconsistent:
LD50 oral (rat): 200–600 mg/kg
LD50 oral (rabbit): 100 mg/kg
LD50 skin (rat): 690 mg/kg.
Health Hazard
Exposures to chlordane cause adverse health effects and poisoning to animals and humans. The acute oral LD50 values of technical grade chlordane for the rat range from 137 to 590 mg/kg and acute dermal LD50 for the rabbit is 1720 mg/kg. Signs of acute chlordane intoxication include ataxia, convulsions, and cyanosis followed by death due to respiratory failure. Rats treated by gavage with 100 mg/kg once a day for 4 days had increased absolute liver weights; fatty infi ltration of the liver; and increased serum triglycerides, creatine phosphokinase, and lactic acid dehydrogenase. Sheep treated by stomach tube with 500 mg/kg showed signs of intoxication, but recovered fully within 5–6 days; a dose of 1000 mg/kg resulted in death after 48 h. Ingestion of chlordane induces vomiting, dry cough, agitation and restlessness, hemorrhagic gastritis, bronchopneumonia, muscle twitching, convulsions, and death among humans. Non-lethal, but accidental poisoning of children has resulted in convulsions, excitability, loss of coordination, dyspnea, and tachycardia. Recovery, however, was complete. Ingestion of chlordane contaminated water (1.2 g/L) caused symptoms of gastrointestinal and neurological disorders. Chronic inhalation of chlordane produced symptoms of poisoning that included, but were not limited to, sinusitis, bronchitis, dermatitis, neuritis, migraine, gastrointestinal distress, fatigue, memory defi cits, personality changes, Exposures to chlordane cause adverse health effects and poisoning to animals and humans. The acute oral LD50 values of technical grade chlordane for the rat range from 137 to 590 mg/kg and acute dermal LD50 for the rabbit is 1720 mg/kg. Signs of acute chlordane intoxication include ataxia, convulsions, and cyanosis followed by death due to respiratory failure. Rats treated by gavage with 100 mg/kg once a day for 4 days had increased absolute liver weights; fatty infi ltration of the liver; and increased serum triglycerides, creatine phosphokinase, and lactic acid dehydrogenase. Sheep treated by stomach tube with 500 mg/kg showed signs of intoxication, but recovered fully within 5–6 days; a dose of 1000 mg/kg resulted in death after 48 h. Ingestion of chlordane induces vomiting, dry cough, agitation and restlessness, hemorrhagic gastritis, bronchopneumonia, muscle twitching, convulsions, and death among humans. Non-lethal, but accidental poisoning of children has resulted in convulsions, excitability, loss of coordination, dyspnea, and tachycardia. Recovery, however, was complete. Ingestion of chlordane contaminated water (1.2 g/L) caused symptoms of gastrointestinal and neurological disorders. Chronic inhalation of chlordane produced symptoms of poisoning that included, but were not limited to, sinusitis, bronchitis, dermatitis, neuritis, migraine, gastrointestinal distress, fatigue, memory defi cits, personality changes, decreased attention span, numbness or paresthesias, blood dyscrasias, disorientation, loss of coordination, dry eyes, and seizures. Chlordane-treated laboratory rats showed blood diseases, including aplastic anemia and acute leukemia
Agricultural Uses
Insecticide: Not approved for use in EU countries. Since 1988, the use and commercial production of chlordane (except for export) has been prohibited in the United States and other countries. The only commercial use still permitted is for fire ant control in power transformers. Chlordane is a broad-spectrum insecticide of the group of polycyclic chlorinated hydrocarbons called cyclodiene insecticides. Chlordane has been used extensively since the 1950s for termite control, as an insecticide for homes and gardens, and as a control for soil insects during the production of crops such as corn. Both the uses and the production volume of chlordane have decreased extensively since the issuance of a registration suspension notice for all food crops and home and garden uses of chlordane by the U.S. Environmental Protection Agency. However, significant commercial use of chlordane for termite control continues. Special groups at risk include children as a result of milk consumed; fishermen and their families because of the high consumption of fish and shellfish, especially freshwater fish; persons living downwind from treated fields; and persons living in houses treated with chlordane pesticide control agents.
Trade name
A SPON-CHLORDANE®; BELT®; CD 68®; CHLORINDAN®; CHLOR KIL®; CHLORODANE®; CORODANE®; CHLORTOX®; DOWCHLOR®[C]; DOW-KLOR®[C]; GOLD CREST®[C]; KILEX LINDANE®; HCS 3260®; KYPCHLOR®; M 140®; M 410®; NIRAN®; OCTACHLOR®; OKTATERR®; OMS 1437®; ORTHO-KLOR®[C]; SD 5532®; SHELL SD-5532®[C]; SYNKLOR®; TAT®; TAT CHLOR® 4; TERMEX®; TOPICHLOR® 20; TOPICLOR®; TOXICHLOR®; VELSICOL® 1068[C]
Safety Profile
Confirmed carcinogen with experimental carcinogenic data. Poison to humans by ingestion and possibly other routes. An experimental poison by ingestion, inhalation, intravenous, and intraperitoneal routes. Moderately toxic by skin contact. Human systemic effects by ingestion or skin contact: tremors, convulsions, excitement, ataxia (loss of muscle coordination), and gastritis. Experimental teratogenic and reproductive effects. Human mutation data reported. Combustible liquid. It is no longer permitted for use as a termiticide in homes. A central nervous system stimulant whose exact mode of action is unknown, but it may involve microsomal enzyme stimulation. Animals poisoned by this and related compounds show an extremely marked loss of appetite and neurological symptoms. The fatal dose to humans is unknown. It has , been estimated to be between 6 and 60 g (0.2 and 2 ounces). One person receiving ~~Oan accidental slan application of 25% solution (amounting to somethmg over 30 g of technical chlordane) developed symptoms withm about 40 minutes and ded, apparently of respiratory failure, before medical attention was obtained. In two patients, death followed exposure to low ingestion doses of chlordane (2-4 g). On microscopic examination, both patients showed severe chronic fatty degeneration of the liver, characteristic of chronic alcoholism. Although these two fatalities cannot be attributed exclusively to chlordane, they are entirely consistent with previous observations that the toxicity of other chlorinated hydrocarbons is much enhanced in the presence of chronic liver damage. The dangerous chronic dose in humans is unknown. small doses exhibit hyperexcitabiltty, tremors, and convulsions, and those that survive long enough show marked anorexia and loss of weight. Symptoms in animals frequently occur withm an hour of the administration of a large dose, but death often is delayed for several days depending on the dosage and route of administration. In any event, symptoms are of longer duration with chlordane than with DDT under similar condtions. are essentially normal, except that the insecticide is found in tissues by means of bioassay. A method for specific, quantitative chemical analysis for chlordane is now available using small amounts of subcutaneous fat. Chronically poisoned animals show degenerative changes in the liver and kidney tubules. emits toxic fumes of Cl-. Experimental animals exposed to repeated Laboratory analyses on poisoned animals When heated to decomposition chlordan
Potential Exposure
Chlordane is a broad spectrum insecticide of the group of polycyclic chlorinated hydrocarbons called cyclodiene insecticides. Chlordane has been used extensively since the 1950s for termite control; as an insecticide for homes and gardens; and as a control for soil insects during the production of crops, such as corn. Both the uses and the production volume of chlordane have decreased extensively since the issuance of a registration suspension notice for all food crops and home and garden uses of chlordane by the United States Environmental Protection Agency. However, significant commercial use of chlordane for termite control continues. Special groups at risk include children as a result of milk consumed; fishermen and their families because of the high consumption of fish and shellfish, especially freshwater fish; persons living downwind from treated fields; and persons living in houses treated with chlordane pesticide control agents.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Speed in removing material from skinis of extreme importance. Shampoo hair promptly if contaminated. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, beginrescue breathing (using universal precautions, includingresuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Carcinogenicity
IARC has concluded that there is inadequate
evidence for carcinogenicity of chlordane to humans and sufficient evidence for its carcinogenicity
to animals.
Chlordane was not mutagenic to bacteria.
Environmental Fate
Biological. In four successive 7-day incubation periods, chlordane (5 and 10 mg/L)
was recalcitrant to degradation in a settled domestic wastewater inoculum (Tabak et al.,
1981).
Soil. The actinomycete, Nocardiopsis sp., isolated from soil extensively degraded pure
cis- and trans-chlordane to dichlorochlordene, oxy-chlordane, heptachlor, heptachlor endoepoxide, chlordene chlorohydrin and 3-hydroxy-trans-chlordane. Oxychlordane is slowly
degraded to 1-hydroxy-2-chlorochlordene (Beeman and Matsumura, 1981). The reported
half-life in soil is approximately one year (Hartley and Kidd, 1987).
The percentage of chlordane remaining in a Congaree sandy loam soil after 14 years
was 40% (Nash and Woolson, 1967).
Chlordane did not degrade in settled domestic wastewater after 28 days (Tabak et al.,
1981).
Plant. Alfalfa plants were sprayed with chlordane at a rate of 1 lb/acre. After 21 days,
95% of the residues had volatilized (Dorough et al., 1972).
Photolytic. Chlordane should not undergo direct photolysis since it does not absorb
UV light at wavelengths greater than 280 nm (Gore et al., 1971).
Chemical/Physical. In an alkaline medium or solvent, carrier, diluent or emulsifier
having an alkaline reaction, chlorine will be released (Windholz et al., 1983). Technical
grade chlordane passed over a 5% platinum catalyst at 200°C resulted in the formation of
tetrahydrodicyclopentadiene (Musoke et al., 1982).
Chlordane (1 mM) in methyl alcohol (30 mL) underwent dechlorination in the presence
of nickel boride (generated by the reaction of nickel chloride and sodium borohydride).
The catalytic dechlorination of chlordane by this method yielded a pentachloro derivative
as the major product having the empirical formula C10H9Cl5 (Dennis and Cooper, 1976).
Chlordane is subject to hydrolysis via the nucleophilic substitution of chlorine by
hydroxyl ions to yield 2,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro-4,7-methano-1Hindene which is resistant to further hydrolysis (Kollig, 1993). The hydrolysis half-life at
pH 7 and 25°C was estimated to be >197,000 years (Ellington et al., 1988).
Emits very toxic fumes of chlorides when heated to decomposition (Lewis, 1990)
Metabolic pathway
Chlordane undergoes a variety of metabolic processes including oxidation, reductive dechlorination, hydrolysis and epoxidation. These reactions afford products involving alteration of the cyclopentane ring; the norbornyl moiety generally remains unaffected except in photochemical reactions when bridged compounds may be formed or dechlorination may occur. Hydroxylation at position 3 is effected by microsomal mixed function oxidases to form 3-hydroxychlordane (12). Dehydration of this compound gives 1,2-dichlorochlordene (13), a key metabolic intermediate in the formation of oxychlordane and other metabolites. A second metabolic pathway involves dehydrochlorination to form heptachlor (2). Dechlorination affords 1-chlorodihydrochlordene (7). Hydrolysis gives 1-chloro-2-hydroxychlordene chlorohydrin (6) which may be metabolised to monochlorodihydroxy and trihydroxy derivatives of dihydrochlordene (Nomeir and Hajjar, 1987).
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working withChlordane you should be trained on its proper handling andstorage. Chlordane must be stored to avoid contact withstrong oxidizers (such as perchlorates, peroxides, permanganates, chlorates, and nitrates), since violent reactions occur.Store in tightly closed containers in a cool, well-ventilatedarea away from heat. A regulated, marked area should beestablished where this chemical is handled, used, or storedin compliance with OSHA Standard 1910.1045.
Shipping
UN2996 Organochlorine pesticides, liquid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Degradation
Acetone-sensitised photolysis of cis-chlordane (1) gave bridged derivatives
to which cage-like structures (19a or 19b) have been assigned. The
isomeric trans-chlordane (1) failed to yield bridged products because the
double bond in these compounds interacts with the endo-chlorine atom
(Fischler and Korte, 1969). However, it was later reported that with transchlordane
(l),bridging did occur forming 20 involving carbon-1 which
has a non-interfering exo-chlorine atom.
Unsensitised photolysis of trans-chlordane (1) in aqueous organic
solvents at wavelengths less than 300 nm gave two isomeric monodechlorinated
products (21a and 2) and ultimately the bis-dechlorination
product (Vollner et al. 1969; Ivie et al. 1972). When cis-chlordane (1) was
irradiated as a solid film, up to 70% was converted after 16-20 hours
irradiation into a mixture of products, more than half of which were
bridged isomers (depending on conditions, the dechlorinated compounds
may also form bridged compounds). These transformations are shown in
Scheme 1.
Chlordane is decomposed by alkalis with the loss of chlorine.
Toxicity evaluation
Technical chlordane is a viscous, amber liquid (bp 175 ?C/267 Pa, vp 1.3 mPa at 25 ?C) soluble in water to about 9 μg/L. It has rat LD50s of 335, 430 (oral), and 840, 690 (dermal) mg/kg. Technical chlordane contains about 60% of the isomers and 10–20% of heptachlor.
Incompatibilities
Contact with strong oxidizers may cause fire and explosions. High heat and contact with alkaline solutions cause decomposition with the production of toxic fumes including chlorine, phosgene, hydrogen chloride. Attacks iron, zinc, plastics, rubber, and coatings.
Waste Disposal
Chlordane is dehydrochlorinated in alkali to form “nontoxic” products, a reaction catalyzed by traces of iron, but the reaction is slow. The environmental hazards of the products are uncertain. Chlordane is completely dechlorinated by sodium in isopropyl alcohol. The UN Recommends incineration methods for disposal of chlordane. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of CHLORDANE
| Melting point: | 106-108℃ |
| Boiling point: | 492.46°C (rough estimate) |
| Density | 1.80 |
| vapor pressure | 10 at 25 °C (Sunshine, 1969)(x 10-6 mmHg) |
| refractive index | nD25 1.56-1.57 |
| Flash point: | 11 °C |
| storage temp. | 0-6°C |
| solubility | Miscible with acetone, cyclohexanone, deodorized kerosene, ethanol, 2-propanol,
trichloroethylene (Worthing and Hance, 1991) |
| Water Solubility | 0.1 mg l-1(25 °C) |
| form | Viscous amber liquid |
| Henry's Law Constant | 4.8 at 25 °C (gas stripping-GC, Warner et al., 1987)(x 10-5 atm?m3/mol) |
| Exposure limits | NIOSH REL: IDLH 0.5 mg/m3, IDLH 100 mg/m3; OSHA PEL: TWA
0.5 mg/m3; ACGIH TLV: TWA 0.5 mg/m3, STEL 2 mg/m3. |
| Stability: | Stable, but readily decomposed by moderately strong alkaline solutions. Corrodes iron and zinc and attacks some types of polymer. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 57-74-9 |
| IARC | 2B (Vol. Sup 7, 53, 79) 2001 |
| EPA Substance Registry System | Chlordane (57-74-9) |
Safety information for CHLORDANE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H351:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for CHLORDANE
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1