Chloranilic acid
Synonym(s):2,5-Dichloro-3,6-dihydroxy-p-benzoquinone;2,5-Dichloro-3,6-dihydroxy-2,5-cyclohexadiene-1,4-dione;NSC 6108;NSC 97383
- CAS NO.:87-88-7
- Empirical Formula: C6H2Cl2O4
- Molecular Weight: 208.98
- MDL number: MFCD00001596
- EINECS: 201-780-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
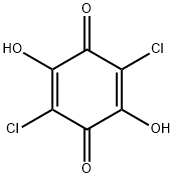
What is Chloranilic acid?
Chemical properties
orange or red crystals or powder
The Uses of Chloranilic acid
Chloranilic acid is the reactant involved in:
Acting as a proton donor in reactions studying dimensionality control
Synthesis of dimethylbipyridyl complexes
Synthesis of (nonylbenzimidazolylmethyl)benzene for preparation of neutral altitudinal rotor-shaped dirhenium metallacycles
Charge-transfer reactions with metoprolol tartrate
Salt formation with organic bases
Synthesis of osmium metalla-rectangles with anticancer activity
The Uses of Chloranilic acid
Chloranilic acid is a strong dibasic organic acid which exhibits electron-acceptor properties on one hand and acidic properties leading to formation of hydrogen bonds on the other hand.
Chloranilic acid is used in spectrophotometry. It reacts with metal ions to form stable complexes. It is also used as a reactant in the preparation of dimethylbipyridyl complexes and (nonylbenzimidazolylmethyl)benzene. Further, it is used in charge-transfer reactions with metoprolol tartrate. It is also employed in the synthesis of osmium metalla-rectangles with anticancer activity. In addition to this, it has potential antibacterial activities against methicillin-resistant Staphylococcus aureus.
The Uses of Chloranilic acid
Reacts with metal cations to form stable salts. Used in spectrophotometry: Hart, organic. Chem. Bull. 33, no. 3 (1961).
What are the applications of Application
Chloranilic acid is Reagent used for the detection and detmination of CA, Sr, Zr, and MO.
Purification Methods
A solution of 8g of quinone in 1L of boiling water is filtered while hot, then extracted twice at about 50o with 200mL portions of *benzene. The aqueous phase is cooled in ice-water. The crystals are filtered off, washed with three 10mL portions of water, and dried at 115o. It can be sublimed in vacuo. [Weissbart & Rysselberghe J Phys Chem 61 765 1957.] The diacetate has m 182-185o [Conant & Fieser J Am Chem Soc 46 1866 1924, Thamer & Voight J Phys Chem 56 225 1952]. [Beilstein 8 IV 2707.]
Properties of Chloranilic acid
| Melting point: | ≥300 °C (lit.) |
| Boiling point: | 300.11°C (rough estimate) |
| Density | 1.93 |
| refractive index | 1.4570 (estimate) |
| storage temp. | Amber Vial, Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | pK1:1.09;pK2:2.42 (25°C) |
| form | Fine Powder |
| color | Orange to red |
| Odor | Odorless |
| Water Solubility | Soluble in hot water and methanol. Insoluble in organic solvents. |
| Merck | 14,2079 |
| BRN | 1875040 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 87-88-7(CAS DataBase Reference) |
| EPA Substance Registry System | 2,5-Cyclohexadiene-1,4-dione, 2,5-dichloro-3,6-dihydroxy- (87-88-7) |
Safety information for Chloranilic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Chloranilic acid
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 87-88-7 CHLORANILLIC ACID 99%View Details
87-88-7 CHLORANILLIC ACID 99%View Details
87-88-7 -
 87-88-7 98%View Details
87-88-7 98%View Details
87-88-7 -
 Chloranilic acid, GR CAS 87-88-7View Details
Chloranilic acid, GR CAS 87-88-7View Details
87-88-7 -
 Chloranilic Acid CAS 87-88-7View Details
Chloranilic Acid CAS 87-88-7View Details
87-88-7 -
 Chloranilic acid 95% CAS 87-88-7View Details
Chloranilic acid 95% CAS 87-88-7View Details
87-88-7 -
 Chloranilic acid 98.00% CAS 87-88-7View Details
Chloranilic acid 98.00% CAS 87-88-7View Details
87-88-7 -
 Chloranilic acid CAS 87-88-7View Details
Chloranilic acid CAS 87-88-7View Details
87-88-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
