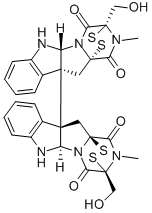
Chaetocin
Synonym(s):(+)-Chaetocin A;EHMT2/G9a Inhibitor II, HMT Inhibitor II, Histone Lysine Methyltransferase Inhibitor II;HMT Inhibitor II, Histone Lysine Methyltransferase Inhibitor II, EHMT2/G9a Inhibitor II;HMTase Inhibitor II, Chaetocin - CAS 28097-03-2 - Calbiochem
- CAS NO.:28097-03-2
- Empirical Formula: C30H28N6O6S4
- Molecular Weight: 696.84
- MDL number: MFCD00133163
- EINECS: 200-258-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-30 15:45:59
What is Chaetocin?
Description
Chaetocin (28097-69-1) is an antimicrobial fungal metabolite. It was found to be a specific inhibitor of the lysine-specific histone methyltransferase SU(VAR)3-9 (IC50 = 0.6 mM) of Drosophila melanogaster and of its human ortholog (IC50 = 0.8 mM). It acts as a competitive inhibitor for S-adenosylmethionine. The specificity of chaetocin for SU(VAR)3-9 makes this compound an excellent tool for the study of heterochromatin-mediated gene repression. Chaetocin has an antimyeloma activity which has been linked to induction of oxidative stress and subsequent apoptosis.
Chemical properties
Chaetocin is supplied as a crystalline solid. A stock solution may be made by dissolving the chaetocin in the solvent of choice, which should be purged with an inert gas. Chaetocin is soluble in organic solvents such as DMSO. The solubility of chaetocin in DMSO is approximately 25 mg/ml.
The Uses of Chaetocin
Chaetocin is an epithiodioxopiperazine antibiotic that has recently shown promise as a selective antitumour agent. Chaetocin is an inhibitor of lysine-specific methyltransferase SU(VAR)3-9 both in vitro and in vivo. Chaetocin is dramatically accumulated in cancer cells via a process inhibited by glutathione. Inside the cell, its activity is mediated by the imposition of oxidative stress.
What are the applications of Application
Chaetocin is a natural metabolite isolated from Chaetomium minutum. It is a nonspecific inhbitor of histone lysine methyltransferases which are important epigenetic enzymes . Induces apoptosis in myeloma cell lines and exhibits antiproliferative activity in mammals. Chaetocin from Chaetomium minutum has been used to determine its effects on sensitization of various cells. It has also been used to determine the biological functions of OS-induced heterochromatin formation.
What are the applications of Application
Chaetocin is a lysine-specific histone methyltransferase inhibitor
General Description
Chaetocin is a fungal metabolite with antimicrobial and cytostatic activity. It belongs to the 3,6-epidithio-diketopiperazines class of which gliotoxin, sporidesmin, aranotin, oryzachloride, verticillin A and the melinacidins are members. Chaetocin is a molecular dimer of two five-membered rings cis fused. Interestingly, the chirality of the 3,6-epidithiodioxopiperazine moiety in chaetocin is opposite to the chirality of gliotoxin, sporidesmin, aranotin, and oryzachloride. Unlike these compounds, chaetocin does not have an antiviral activity. This fungal toxin showed strong cytotoxicity against HeLa cells (IC50 = 0.05 mg/ml).
Biochem/physiol Actions
Chaetocin is a competitive inhibitor for S-adenosylmethionine. The specificity of chaetocin for SU(VAR)3-9 makes this compound an excellent tool for the study of heterochromatin-mediated gene repression.
storage
-20°C
References
1) Greiner et al. (2005), Identification of a specific inhibitor of the histone methyltransferase SU(VAR)3-9; Nat. Chem. Biol., 1 143
2) Isham et al. (2007), Chaetocin: a promising new antimyeloma agent with in vitro and in vivo activity mediated via imposition of oxidative stress; Blood, 109 2579
Properties of Chaetocin
| Density | 1.87±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (up to 25 mg/ml) or in Ethanol (up to 25 mg/ml). |
| pka | 12.39±0.10(Predicted) |
| form | White to off-white solid |
| color | White |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
Safety information for Chaetocin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Chaetocin
| InChIKey | PZPPOCZWRGNKIR-PNVYSBBASA-N |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![PHENOL, 2-METHOXY-5-[2-(3,4,5-TRIMETHOXYPHENYL)ETHYL]-](https://img.chemicalbook.in/CAS/GIF/95041-90-0.gif)

You may like
-
 Chaetocin from Chaetomium minutum CAS 28097-03-2View Details
Chaetocin from Chaetomium minutum CAS 28097-03-2View Details
28097-03-2 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1