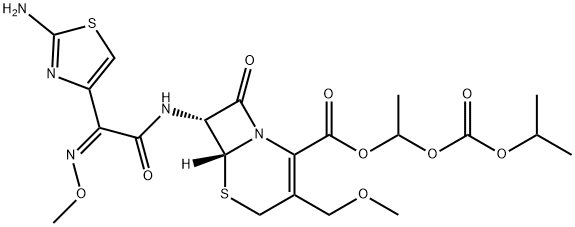
Cefpodoxime
Synonym(s):(6R,7R)-7-{2-(2-Amino-4-thiazolyl)-2-[(Z)-methoxyimino]acetamido}-3-(methoxymethyl)-3-cephem-4-carboxylate;Cefpodoxime free acid
- CAS NO.:80210-62-4
- Empirical Formula: C15H17N5O6S2
- Molecular Weight: 427.46
- MDL number: MFCD00864906
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-21 22:41:43

What is Cefpodoxime?
Absorption
Cefpodoxime proxetil is a prodrug that is absorbed from the gastrointestinal tract and de-esterified to its active metabolite, cefpodoxime. Following oral administration of 100 mg of cefpodoxime proxetil to fasting subjects, approximately 50% of the administered cefpodoxime dose was absorbed systemically.
Chemical properties
Off-White Solid
The Uses of Cefpodoxime
A metabolite of Cefpodoxime Proxetil (C243860). An antibacterial.
The Uses of Cefpodoxime
Cefpodoxime Proxetil metabolite antibiotic
The Uses of Cefpodoxime
A metabolite of Cefpodoxime Proxetil. An antibacterial.
Background
Cefpodoxime is an oral third generation cephalosporin antibiotic with effectiveness against most Gram positive and Gram negative bacteria. Commonly used to treat acute otitis media, pharyngitis, and sinusitis, cefpodoxime proxetil is a prodrug which is absorbed and de-esterified by the intestinal mucosa to Cefpodoxime.
Indications
Indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms.
Definition
ChEBI: A third-generation cephalosporin antibiotic with methoxymethyl and (2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetamino substituents at positions 3 and 7, respectively, of the cephem skeleton. Given by mouth as its proxetil ester prodrug, it is used to treat acute otitis media, pharyngitis, and sinusitis.
What are the applications of Application
Cefpodoxime, Free Acid is a metabolite of Cefpodoxime Proxetil and an anti-bacterial
brand name
Vantin (Pharmacia & Upjohn).
Antimicrobial activity
It is stable to a wide range of plasmid-mediated β-lactamases. It induces the chromosomal β-lactamases of Ps. aeruginosa, Enterobacter spp., S. marcescens and Citrobacter spp., but is a less potent inducer than cefoxitin.
Biological Activity
cefpodoxime, as known as r 3763, is a metabolite of cefpodoxime proxetil. it is demonstrated that cefpodoxime, as an oral third generation cephalosporin antibiotic, is active against most gram-positive and gram-negative bacteria.cefpodoxime suppresses bacterial septum and cell wall synthesis by binding to penicillin-binding proteins (pbps) located in the bacterial cytoplasmic membrane.
Pharmacokinetics
Oral absorption: c. 50%
Cmax 200 mg oral: 2.1 mg/L after 3 h
Plasma half-life: c. 2.2 h
Volume of distribution: c. 35 L
Plasma protein binding: 20–30%
Absorption and distribution
The ester is rapidly hydrolyzed to the parent compound in the small intestine. Bioavailability increases to 65% if taken with food, but antacids and H2-receptor antagonists reduce absorption. Unabsorbed drug is hydrolyzed and excreted in the feces.
It is well distributed and penetrates well into tissues (including lung tissue) and inflammatory exudate to achieve concentrations inhibitory to common pathogens.
Metabolism and excretion
The hydrolyzed prodrug is not subject to further metabolism. About 80% of the absorbed compound (30–40% of the original dose) appears in the urine over 24 h. Excretion is by glomerular filtration and tubular secretion; probenecid delays secretion and increases the peak plasma concentration.
Metabolism and excretion
The hydrolyzed prodrug is not subject to further metabolism. About 80% of the absorbed compound (30–40% of the original dose) appears in the urine over 24 h. Excretion is by glomerular filtration and tubular secretion; probenecid delays secretion and increases the peak plasma concentration.
Pharmacokinetics
Cefpodoxime is shown to be effective against most Gram positive and Gram negative bacteria, except Pseudomonas aeruginosa, Enterococcus, and Bacteroides fragilis.
Clinical Use
Cefpodoxime has been used principally for the treatment of upper and lower respiratory tract infections in children and adults.
Side Effects
The drug is well tolerated, but gastrointestinal disturbance with diarrhea is common. Pseudomembranous colitis has been reported occasionally. Other side effects are those common to cephalosporins.
in vitro
cefpodoxime showed antibacterial activities against obligatory anaerobes and salmonella spp., shigella spp. and neisseria meningitides. the activity of cefpodoxime was less active than r95867, an active form of cs-834, against gram-negative bacteria [1]. cefpodoxime was quite stable to hydrolysis by β-lactamases produced from b. cereus and e. coli hb101/pbr322 [2].
in vivo
male ddy mice were administered orally in a volume of 0.2 ml of 0.5% carboxymethyl cellulose sodium salt. after 7 days, it was shown that cefpodoxime had good efficacy against streptococcus spp. and k. pneumoniae infection in mice [1].
Metabolism
Not Available
References
[1]. sakagawa, e., otsuki, m., oh, t., & nishino, t. in-vitro and in-vivo antibacterial activities of cs-834, a new oral carbapenem. journal of antimicrobial chemotherapy, 1998; 42: 426-437.
[2]. fukuoka, t., ohya, s., utsui, y., domon, h., takenouchi, t., koga, t., … kuwahara, s. in vitro and in vivo antibacterial activities of cs-834, a novel oral carbapenem. antimicrobial agents and chemotherapy, 1997; 41(12): 2652–2663.
Properties of Cefpodoxime
| Melting point: | 200-202°C |
| Density | 1.78±0.1 g/cm3(Predicted) |
| RTECS | XI0367365 |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Soluble in DMSO |
| pka | 2.77±0.50(Predicted) |
| form | neat |
| form | Solid |
| color | White to off-white |
| Sensitive | Light Sensitive |
| BRN | 6021381 |
| Stability: | Hygroscopic |
Safety information for Cefpodoxime
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P342+P311:IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. |
Computed Descriptors for Cefpodoxime
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 80210-62-4 Cefpodoxime Proxetil EP Impurity A; Cefpodoxime Acid 98%View Details
80210-62-4 Cefpodoxime Proxetil EP Impurity A; Cefpodoxime Acid 98%View Details
80210-62-4 -
 80210-62-4 98%View Details
80210-62-4 98%View Details
80210-62-4 -
 Cefpodoxime CAS 80210-62-4View Details
Cefpodoxime CAS 80210-62-4View Details
80210-62-4 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0