Cefovecin sodium
- CAS NO.:141195-77-9
- Empirical Formula: C17H18N5NaO6S2
- Molecular Weight: 0
- MDL number: MFCD31562853
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-08 09:17:02
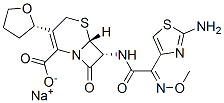
What is Cefovecin sodium?
Description
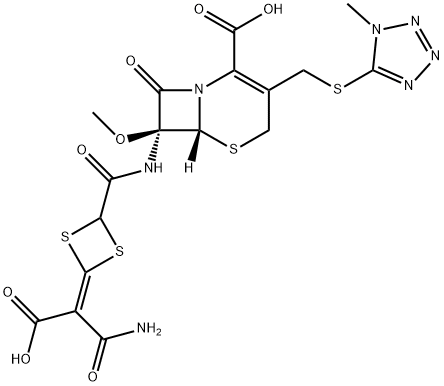
Cefovecin sodium is a semi-synthetic broad-spectrum antibacterial agent from the cephalosporin class of chemotherapeutic agents. Cefovecin is the non-proprietary designation for (6R,7R)-7- [[(2Z)-(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-8-oxo-3-[(2S)-tetrahydro-2-furanyl]-5-thia-1- azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, monosodium salt.
Cefovecin Sodium is an antibiotic of the cephalosporin class. It is used for the treatment of skin infections caused by Pasturella multocida in cats, and Staphylococcus intermedius/S. canis in dogs. Cefovecin Sodium is the first single-dose injectable antibiotic for dogs and cats.
The Uses of Cefovecin sodium
Sodium salt of Cefovecin, a cephalosporin antibiotic used in the treatment of skin conditions in dogs and cats.
Indications
Dogs
Cefovecin sodium is indicated for the treatment of skin infections (secondary superficial pyoderma, abscesses,
and wounds) in dogs caused by susceptible strains of Staphylococcus intermedius and Streptococcus canis (Group G).
Cats
Cefovecin sodium is indicated for the treatment of skin infections (wounds and abscesses) in cats caused by susceptible strains of Pasteurella multocida.
Definition
ChEBI: Cefovecin sodium is an organic molecular entity.
Side Effects
In cats, the most common effects are vomiting, diarrhea, decreased appetite, lethargy, hyperactivity or “acting strange” and inappropriate urination.
In dogs, the most common adverse effects were lethargy, decreased appetite, vomiting, diarrhea, blood in feces, dehydration, and flatulence.
Safety
Dogs
CONVENIA administered to healthy four month old dogs at doses of 12 mg/kg (1.5 X), 36 mg/kg (4.5 X), and 60 mg/kg (7.5 X) every seven days by dorsoscapular subcutaneous injections was well-tolerated for a total of 5 doses. Vomiting and diarrhea were seen in all treatment groups, with the incidence of vomiting and the incidence and duration of diarrhea increasing in a dose-related manner. Injection site irritation and transient edema occurred with increasing frequency in a dose-related manner and with repeat injections. Two injection site reactions included a seroma over the shoulder and swelling lasting > 30 days. Dogs dosed at 36 mg/kg had a significant (p = 0.0088) increase in BUN (all means remained within the normal range) compared to the controls. One dog dosed at 60 mg/kg exhibited a glomerulopathy on histopathology, and one dog in this same group had minimal peliosis hepatis. At an exaggerated dose of 180 mg/kg (22.5X) in dogs, CONVENIA caused some injection site irritation, vocalization and edema. Edema resolved within 8-24 hours.
www.zoetisus.com
Properties of Cefovecin sodium
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
Safety information for Cefovecin sodium
Computed Descriptors for Cefovecin sodium
| InChIKey | QRIBXVGHDLFNNE-RUPIGQPYNA-M |
| SMILES | [Na+].N12[C@@]([H])([C@H](NC(/C(/C3=CSC(N)=N3)=N/OC)=O)C1=O)SCC([C@@H]1CCCO1)=C2C([O-])=O |&1:2,4,23,r| |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1