Cefmetazole
- CAS NO.:56796-20-4
- Empirical Formula: C15H17N7O5S3
- Molecular Weight: 471.53
- MDL number: MFCD00865068
- EINECS: 260-384-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-29 14:36:51
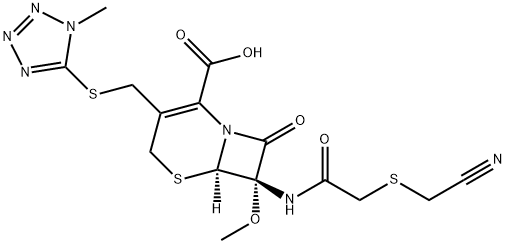
What is Cefmetazole?
Absorption
Bioavailability is approximately 100% following intramuscular injection.
Toxicity
Oral LD50 in rats is 3,204 mg/kg. With other b-lactam antibiotics, adverse effects following overdosage have included nausea, vomiting, epigastric distress, diarrhea, and convulsions.
Description
Cefmetazole is a cephamycin with a nonaromatic side chain at C-7. In addition to a fairly characteristic second-generation cephalosporin antimicrobial spectrum, it possess fairly significant anti-antiaerobic activity. The ejection of its C-3 side chain leads to alcohol intolerance of the disulfuram type and prolonged clotting times.
General Description
Cefmetazole was synthesized by Sankyo Co. in 1976starting with a biosynthetic cephamycin. It shows excellent activity against Serratia and Proteus, against which cefazolin is not active, and it has stronger activity than cefoxitin, another derivative of cephamycin. Cefmetazole is active against anaerobes and resistant to β-lactamase, but it is not active against Pseudomonas aeruginosa.
Chemical properties
White Solid
The Uses of Cefmetazole
Semi-synthetic antibiotic derived from Cephamycin. Antibacterial.
The Uses of Cefmetazole
A broad spectrum second generation cephalosporin antibiotic
What are the applications of Application
Cefmetazole is a semi-synthetic antibiotic
Indications
For the treatment of infections caused by susceptible organisms.
Background
A semisynthetic cephamycin antibiotic with a broad spectrum of activity against both gram-positive and gram-negative microorganisms. It has a high rate of efficacy in many types of infection and to date no severe side effects have been noted.
Definition
ChEBI: A cephalosporin antibiotic containg an N1-methyltetrazol-5-ylthiomethyl side-chain at C-3 of the parent cephem bicyclic structure.
Antimicrobial activity
It is active
against Pr. mirabilis, Pr. vulgaris, Morganella morganii, Yersinia spp.and most anaerobes. S. marcescens is moderately susceptible,
but Ps. aeruginosa and E. faecalis are resistant. It is active against
Mycobacterium fortuitum and some strains of M. chelonei. It is
resistant to a wide range of β-lactamases.
The serum concentration at the end of a 1 g intravenous
infusion is around 77 mg/L. Plasma protein binding is 68%. It
is principally excreted in the urine with a plasma half-life of c.
1.3 h; 70% is recovered over the first 6 h. In patients whose
creatinine clearance is less than 10 mL/min, plasma levels are
elevated and the plasma half-life is increased to around 15 h.
Side effects associated with the methylthiotetrazole group
at position C-3 have been reported. Uses are similar to those
of cefoxitin, but it is not widely available.
Pharmacokinetics
Cefmetazole is a second-generation cephalosporin. The cephalosporins are bactericidal drugs with both gram-positive and gram-negative activity. They inhibit bacterial cell wall synthesis in a way similar to the penicillins. Cefmetazole is more active than 1st-generation cephalosporins against indole-positive Proteus, Serratia, anaerobic gram-negative bacilli (including B. fragilis), and some E. coli, Klebsiella, and P. mirabilis, but is less active than cefoxitin or cefotetan against most gram-negative bacilli.
Metabolism
No appreciable metabolism.
Properties of Cefmetazole
| Melting point: | 163-165?C |
| Density | 1.4380 (rough estimate) |
| refractive index | 1.7400 (estimate) |
| storage temp. | 2-8°C |
| solubility | Methanol (Slightly, Heated) |
| pka | 2.65±0.50(Predicted) |
| form | Solid |
| color | White |
| Water Solubility | Soluble in water |
| CAS DataBase Reference | 56796-20-4(CAS DataBase Reference) |
Safety information for Cefmetazole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P284:Wear respiratory protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Cefmetazole
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![1-[[(6R,7R)-7-[[(2Z)-(2-Amino-4-thiazolyl)(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-5,6,7,8-tetrahydro-quinolinium inner salt](https://img.chemicalbook.in/CAS/GIF/84957-30-2.gif)


You may like
-
 Cefmetazole 95.00% CAS 56796-20-4View Details
Cefmetazole 95.00% CAS 56796-20-4View Details
56796-20-4 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1