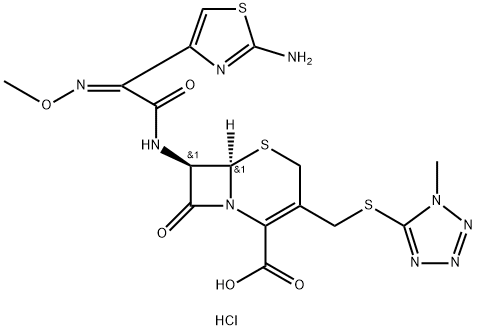
Cefmenoxime
- CAS NO.:65085-01-0
- Empirical Formula: C16H17N9O5S3
- Molecular Weight: 511.56
- MDL number: MFCD00864851
- EINECS: 278-299-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-30 18:52:02
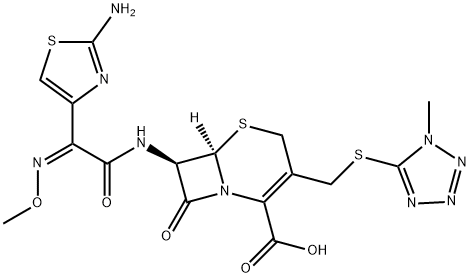
What is Cefmenoxime?
Absorption
Bioavailability is approximately 100% following intramuscular injection.
Toxicity
Information on cefmenoxime overdosage in humans is not available. However, with other b-lactam antibiotics, adverse effects following overdosage have included nausea, vomiting, epigastric distress, diarrhea, and convulsions.
Originator
Tacef,Takeda,W. Germany,1983
The Uses of Cefmenoxime
Cefmenoxime (cas# 65085-01-0) is a compound useful in organic synthesis.
The Uses of Cefmenoxime
Antibacterial.
Indications
Used to treat female gynecologic and obstetric infections caused by susceptible aerobic (including the gonococcus) and anaerobic bacteria.
Background
Cefmenoxime is a novel broad-spectrum and third-generation cephalosporin antibiotic that is typically used in the treatment of female gynecologic and obstetric infections. It is reported to exhibit high activity against a wide variety of gram-positive and gram-negative bacteria.
Definition
ChEBI: A third-generation cephalosporin antibiotic, bearing a 2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino group at the 7beta-position and a [(1-methyl-1H-tetrazol-5-yl)sulfanyl]methyl group at the 3-position.
Manufacturing Process
7β-[α-Methoxyimino-α-(2-aminothiazol-4-yl)acetamido]cephalosporanicacid trifluoroacetic acid salt is dissolved in a solution of 272 mg of 1-methyl-5- mercapto-1H-tetrazole, 555 mg of sodium bicarbonate and 68 mg of triethylbenzylammonium bromide in 10 ml of water. The solution is heated at 60°C in nitrogen atmosphere for 6 hours. After cooling, the reaction solution is passed through a column of Amberlite XAD-2 and eluted with water and then with 2.5% ethanol. The procedure yields sodium 7β-[α-methoxyimino-α- (2-aminothiazol-4-yl)acetamido]-3-(1-methyl-1H-tetrazol-5-ylthiomethyl)-3- cephem-4-carboxylate, MP 174°C to 175°C (decomposition).
brand name
Cefmax (TAP).
Therapeutic Function
Antibacterial
Antimicrobial activity
A semisynthetic cephalosporin supplied as the hydrochloride.
Its activity is very similar to that of cefotaxime. A
500 mg intramuscular injection achieves a plasma concentration
of 15 mg/L after 40 min. A concentration of 200 mg/L is
attained after intravenous administration of 1 g. The plasma
half-life is c. 1 h. Around 77% is protein bound. Probenecid
increases peak plasma levels and extends the plasma half-life
to 1.8 h. Therapeutic concentrations are achieved in CSF.
There is a degradation product with a long half-life (around
40 h), but 80–92% of the drug is recovered unchanged from
the urine. In patients with renal insufficiency, no significant
relation was found between creatinine clearance and peak
serum concentrations but there was a linear relationship
with plasma half-life and total body clearance. About 10%
of the dose appears in the feces, mostly extensively degraded,
possibly
by the fecal flora.
Toxicity, side effects and clinical use are those common to
group 4 cephalosporins.
Pharmacokinetics
Cefmenoxime is a semisynthetic beta-lactam cephalosporin antibiotic with activity similar to that of cefotaxime. It has broad spectrum activity against Gram positive and Gram negative bacteria.
Metabolism
Not appreciably metabolized.
Properties of Cefmenoxime
| Density | 1.96±0.1 g/cm3(Predicted) |
| pka | 2.61±0.50(Predicted) |
| CAS DataBase Reference | 65085-01-0(CAS DataBase Reference) |
Safety information for Cefmenoxime
Computed Descriptors for Cefmenoxime
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

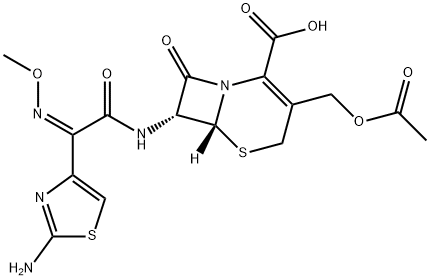
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1